Imaging of postoperative internal hernias
Images
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
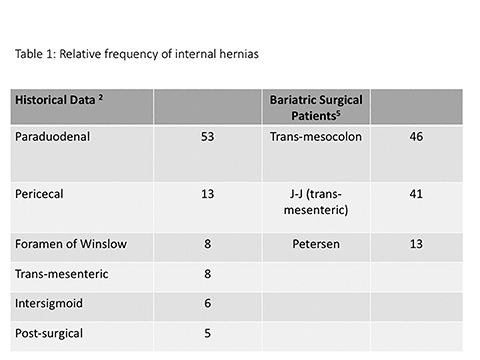
Internal hernias remain one of the most challenging and potentially frustrating diagnoses that face abdominal imagers.1 Before the advent of Roux-en-Y gastric bypass (RYBG) surgery, the paraduodenal hernia was usually reported as the most common internal hernia, accounting for more than 50% of cases (Table 1).2 However, postsurgical internal hernias have now become much more common, attributable to the increasing popularity of bariatric surgery during the mid-2000’s.1,3-5 In postoperative bariatric patients, internal hernias are one of the more common postoperative complications, reported in 2-3% of patients.6,7 Hernias following RYGB surgery have a high risk of torsion and bowel ischemia, and they require prompt recognition and surgical treatment.8 Unfortunately, despite advances in multi-detector CT technology and routine use of multi-planar reconstruction techniques, the radiological diagnosis of any internal hernia remains difficult, and is further hampered by the complexity of post-bariatric-surgical anatomy. Understanding the RYGB surgical approach and its appearance at postsurgical CT, as well as the types of postoperative internal hernia and their common imaging features can assist radiologists in making the diagnosis of postoperative internal hernia.
RYGB: rationale and technique
Gastric bypass surgery is now most commonly performed laparoscopically, and uses a combination of restrictive and malabsorptive components to result in patient weight loss. While most postoperative weight loss was originally thought to arise from early satiety resulting from the small gastric remnant, there is increasing realization that weight loss is related to a combination of factors, including complex changes in metabolism that are beyond the scope of this article.3,9
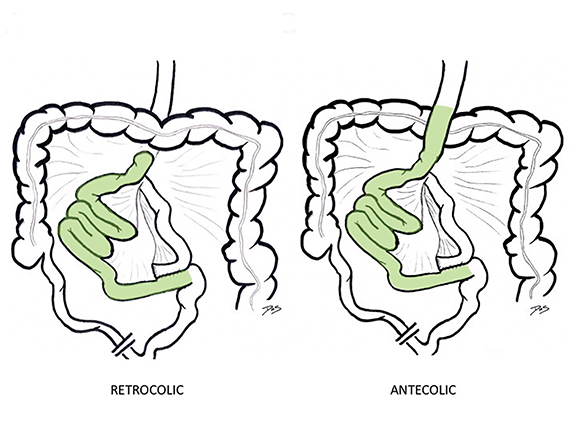
The traditional RYGB surgical approach begins with fashioning a small gastric pouch using the gastric fundus, leaving the larger excluded stomach intact. 10,11 The jejunum is divided 25-50 cm distal to the ligament of Treitz, with the distal component brought up and anastomosed to the gastric remnant (the G-J anastomosis), creating the roux limb (also known as the efferent or alimentary limb). The roux limb may be brought up over (antecolic) or through (retrocolic) the transverse colon mesentery (Figure 1). Finally, the biliopancreatic or afferent limb is anastomosed 75-150 cm distal to the G-J anastomosis in side-to-side fashion (the enteroenterostomy or J-J anastomosis). While RYGB remains a relatively common procedure in the U.S., other bariatric surgical procedures like the laparoscopic sleeve gastrectomy have become increasingly popular in recent years due to their comparable efficacy and lower reported incidence of complications, including internal hernias.12
RYGB: imaging technique and normal postoperative appearance
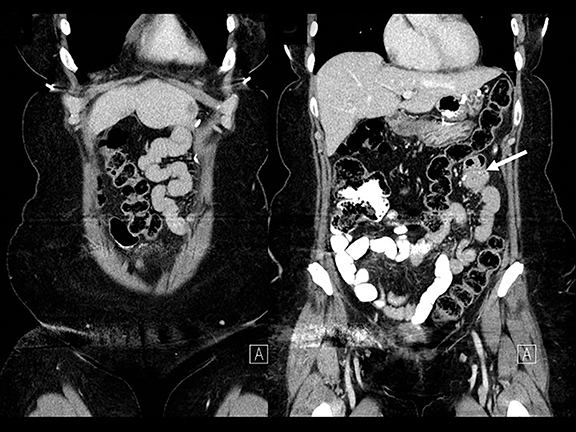
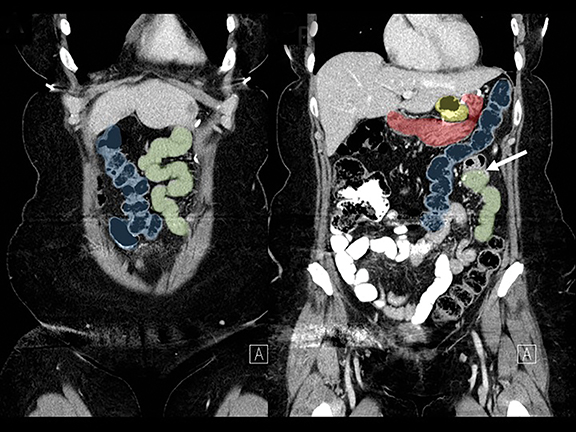
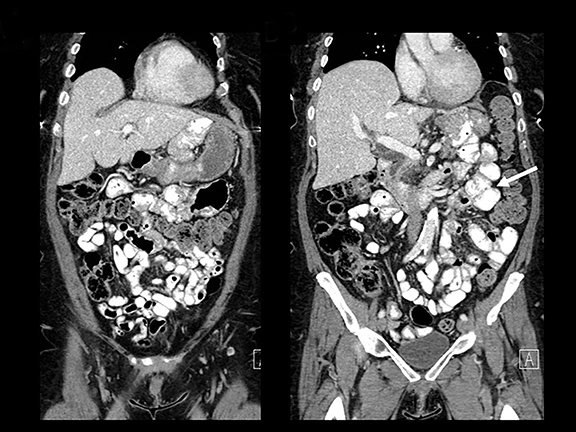
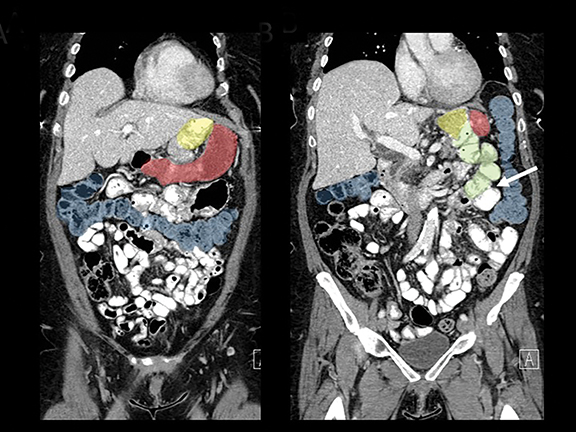
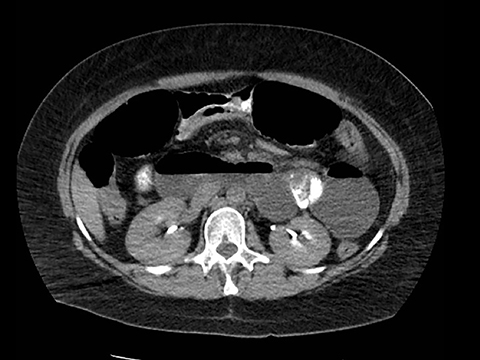
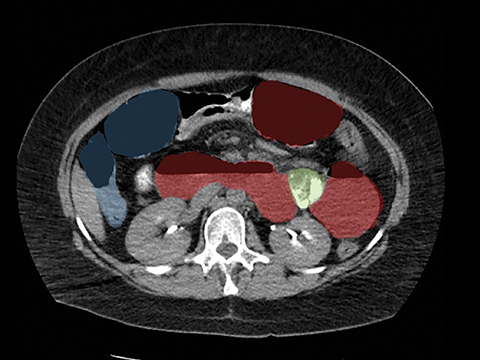
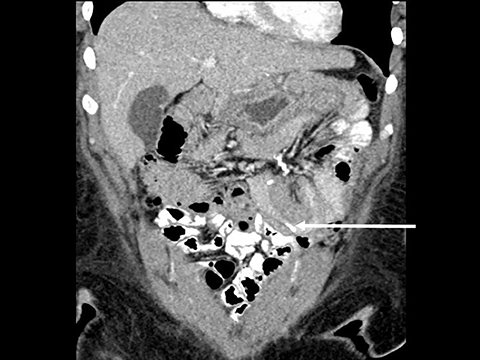
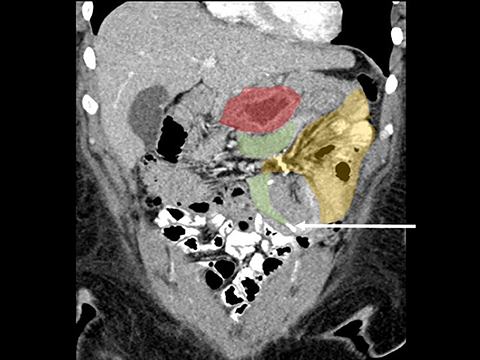
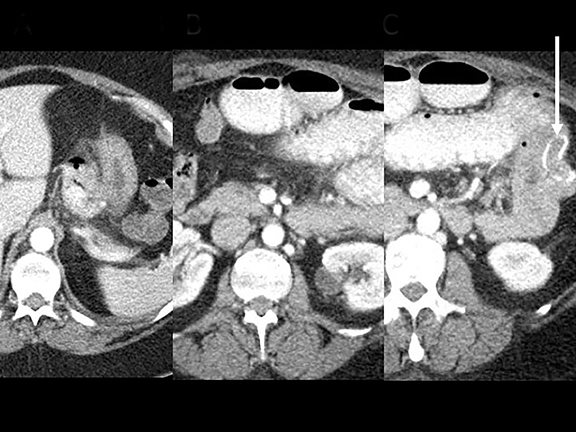
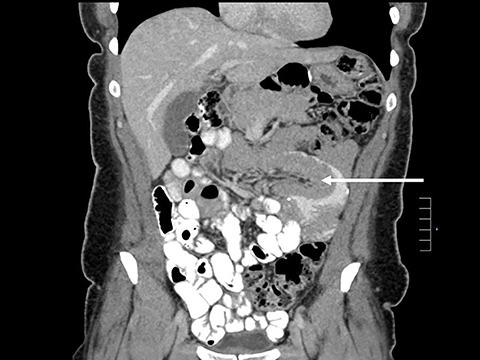
In the immediate postoperative period when an anastomotic leak is suspected, traditional fluoroscopy studies with water-soluble contrast agents are usually employed.13 Although fluoroscopic GI evaluations can also demonstrate postoperative internal hernias, CT is the mainstay for diagnosis.14 As many patients with suspected internal hernia present to the emergency department, imaging is often performed without oral contrast material. However, when possible, administering a small volume of oral contrast material immediately prior to the CT can facilitate identification of the roux limb.5,10 In addition to an opacified roux limb, multiplanar reconstructions are invaluable for understanding the patient’s postoperative anatomy, showing the anterior course of the roux limb with antecolic bypass (Figures 2,3) and more posterior location with retrocolic bypass (Figures 4,5). Both axial and reconstructed images readily depict the location of the J-J anastomosis, seen in the left mid-abdomen in normal postoperative patients (arrows, Figures 2-5). Use of intravenous contrast is preferred when possible, as it facilitates identification of mesenteric vasculature and highlights areas of decreased bowel wall perfusion in the setting of ischemia.15
RYBG Internal hernias
Clinical presentation
Patient signs and symptoms from internal hernias are nonspecific. While some hernias are completely asymptomatic, patient complaints often range from intermittent vague abdominal discomfort to vomiting and acute abdominal pain.5,6,10 Although patients often present with symptoms of small bowel obstruction, compared to non-gastric bypass patients, vomiting may be less common, likely related to the small size of the gastric pouch. Time to postoperative presentation of internal hernias is quite variable, occasionally occurring in the immediate postoperative period, but averaging 10-14 months after surgery.6,7 As patient symptoms and time of presentation can overlap with other common postoperative complications (including adhesive bands, ulcers, anastomotic narrowing, and cholelithiasis), accurate clinical diagnosis is often difficult.5,10,11
Classification
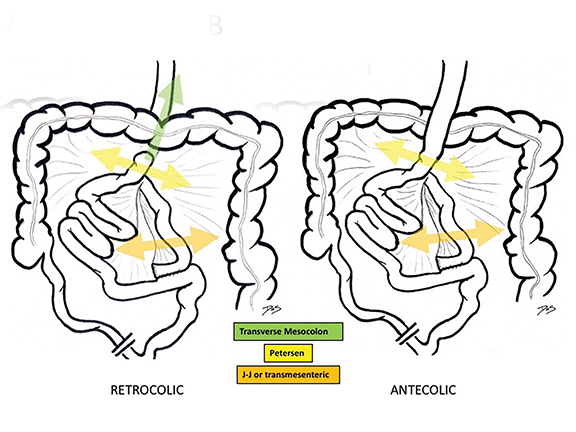
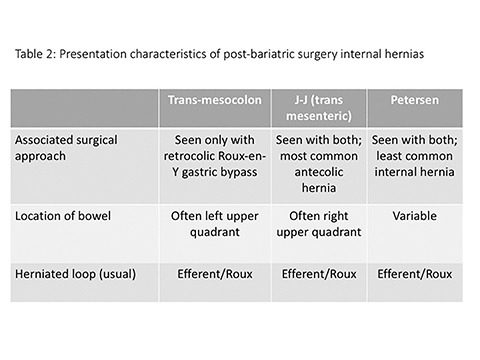
There are three main types of RYGB-associated postoperative internal hernias: transverse mesocolonic hernias, which extend through the transverse mesocolon; J-J hernias, which traverse through the small-bowel mesenteric defect near the enteroenterostomy; and Petersen hernias, which course through the potential space between the roux limb and the transverse mesocolon (Figure 6). While the transverse mesocolonic hernia is seen only in patients with a retrocolic roux limb, the other two hernias are seen in both the retro- and antecolic roux limb surgeries.
While variable, most series suggest that trans-mesocolonic hernias are most common, particularly in patients with a retrocolic surgical approach (Table 2).7 As antecolic surgical approaches avoid a mesocolonic defect, J-J hernias have a higher incidence in this surgical population. Petersen hernias, although reportedly common in some small series, are likely least common overall.1,7
Variability in the incidence of the three subtypes of post-bariatric internal hernias over time likely relates in part to the evolving RYGB surgical approach. Initial open RYGB procedures had a much lower incidence of postoperative hernias, attributable to fixation of bowel loops from adhesions associated with open surgical approaches.6 In laparoscopic RYGB, intra-operative closure of any potential hernia sites with nonabsorbable continuous sutures, as well as migration to antecolic bypass methods has been adopted by many surgeons in an attempt to decrease postoperative hernias.6 However, despite these alterations in surgical techniques, internal hernias remain relatively common, potentially related to progressive postoperative loss of mesenteric fat, and resultant enlarging mesenteric defects.7
Keys to diagnosis
While internal hernias are challenging to identify, several key findings are suggestive of the diagnosis: dilated small-bowel loops (may be pancreaticobiliary, roux, or distal loops); displacement of the J-J anastomosis out of the left mid-abdomen; clustered small-bowel loops, often in the left upper quadrant (but potentially anywhere in the abdomen); swirling of the mesentery (seen with associated torsion); and convergence of bowel loops and vessels, potentially with associated mesenteric edema (seen at the site of herniation). Of these, swirling of the mesentery has been shown to be most sensitive in one series.1 Displacement of the JJ anastomosis, bowel loops located behind the superior mesenteric artery, and crowding of mesenteric vasculature at the hernia site (among other findings) have been shown to be specific but not very sensitive for diagnosis of internal hernias. In general, however, the more findings that are present, the more likely the diagnosis of internal hernia.
Subtypes and imaging features
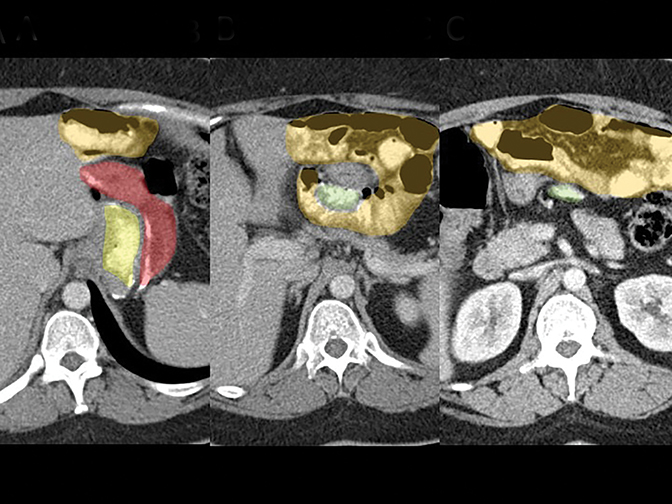
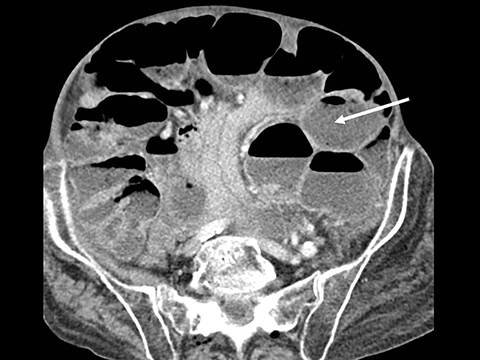
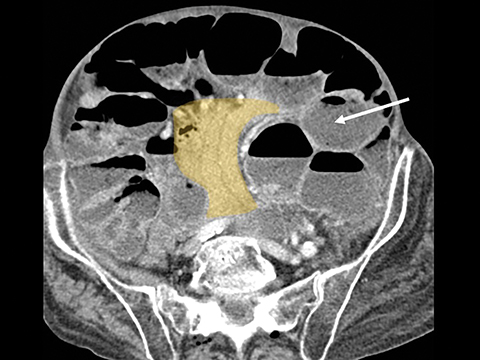
Trans-mesocolonic hernia—Trans-mesocolonic hernias occur through the defect in the transverse mesocolon created for passage of the retrocolic roux limb. Thus, herniated bowel loops typically reside in the left upper quadrant, sometimes causing mass effect on the gastric remnant. Although only occurring in patients with retrocolic RYGB surgeries, as noted previously, these hernias account for a large proportion of internal hernias in RYGB patients. The relatively small aperture associated with this type of hernia is thought to explain the high risk of torsion and ischemia. (Figures 7 and 8).
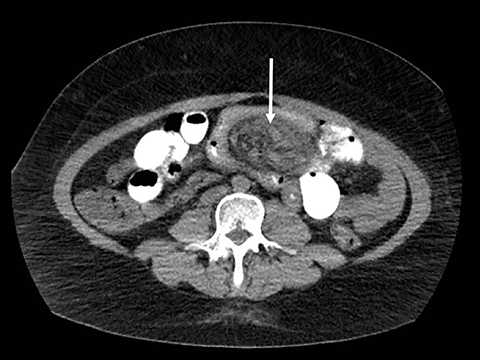
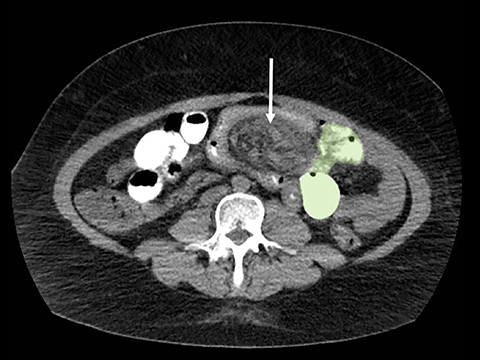
J-J hernia—J-J hernias (enteroenterostomy or trans-mesenteric hernias) occur when bowel loops traverse the repaired mesenteric defect at the site of the jejunal to jejunal anastomosis. For antecolic RYGB, these likely represent the most common type of internal hernia. While bowel loops may traverse left-to-right or right-to-left, most commonly the herniated bowel resides in the right upper quadrant. Although displacement of the J-J anastomosis out of the left mid-abdomen may be seen with any internal hernia, it has been our anecdotal experience that this is more common with J-J hernias. (Figures 9-12).
Petersen hernia—The Petersen hernia probably represents the least common RYGB-associated hernia, created when bowel loops move into the potential space between the transverse mesocolon and the antecolic or retrocolic roux loop. Petersen hernias may occur alone or, in patients with a retrocolic bypass, in combination with trans-mesocolonic hernias. Like other internal hernias, clinical presentations can be variable. However, time to presentation may be longer with Petersen hernias.16 While CT findings can mimic other internal hernias including bowel obstruction and swirling mesentery/vessels, location of bowel loops in the left upper quadrant is more commonly seen with this type of hernia.16 (Figures 13,14)
Differential diagnosis of internal hernias
Patients can present with several other postoperative complications following RYGB, some of which demonstrate nonspecific symptoms similar to internal hernias. A relatively common early complication is the postoperative leak, most occurring at the G-J anastomosis.17 Leaks are usually easily depicted at a UGI exam, but also are readily demonstrated at CT. While transient anastomotic narrowing can occur secondary to perioperative edema, these usually resolve quickly and may not require imaging. Jejunitis and frank jejunal ischemia may also develop in the acute perioperative period, usually well-depicted at IV contrast enhanced CT. Chronic strictures are more common at the G-J than the J-J anastomosis and can result in obstructive symptoms and classic findings at CT (Figure 15). More chronic complications include marginal ulcers, typically presenting at the G-J anastomosis, likely related to exposure of the jejunal limb to gastric acid. While potentially seen at CT, these are more often identified endoscopically. Intussusceptions typically occur near the J-J anastomosis and may be obstructive or transient and incidental (Figure 16). Finally, adhesive bands are common complications of laparoscopic surgical approaches, and can present with imaging findings mimicking internal hernias.8 As adhesive bands may be associated with similar serious complications to internal hernias (i.e., closed-loop obstruction or bowel ischemia), misdiagnosis as an internal hernia is of uncertain significance.
Non-postoperative internal hernias: Common features
In the absence of bariatric surgery, several other types of internal hernias may be encountered, often classified in three broad categories: normal orifice, unusual fossae or recesses, and trans-mesenteric. Hernias through a normal orifice include lesser sac hernias through the foramen of Winslow, as well as paraduodenal hernias through the fossae of Waldeyer and Landzert. Hernias through unusual fossae or recesses include paracecal hernias and result in displacement of the cecum anteriorly and medially. Transmesenteric hernias, commonly associated with volvulus, occur through congenital or acquired defects, most often near the terminal ileum or ligament of Treitz (Figures 17, 18). Regardless of type, internal hernias may result in closed loop obstruction – seen at CT as a C-shaped loop of distended bowel, with stretched/thickened mesenteric vessels extending toward the point of obstruction. Finding the hernia orifice may be challenging but, like RYGB hernias, is often facilitated by review of multiplanar reformatted images, which may better depict convergence of bowel and mesenteric fat extending through the defect. As is the case with bariatric surgery-related hernias, closed loop obstructions are often associated with bowel ischemia and usually require prompt surgical intervention.
Conclusion
Internal hernias are common complications in the postoperative bariatric patient. There should be a high index of suspicion for an internal hernia, particularly if there is evidence of prior gastric bypass, dilated small bowel loops, and a mesenteric swirl. Even in non-bariatric patients, once a small-bowel obstruction is detected and an internal hernia is suspected, key features to look for include convergence of mesenteric vessels, displaced bowel loops, or presence of closed-loop obstruction and/or evidence of ischemia warranting surgical intervention.
References
- Lockhart ME, Tessler FN, Canon CL, et al. Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am J Roetntgenol. 2007;188(3):745-750.
- Takeyama N, Gokan T, Ohgigya Y, et al. CT of internal hernias. Radiographics. 2005;25(4):997-1015.
- Menzo EL, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand J Surg. 2015;104 (1): 18-23.
- Johnson EE, Simpson NS, Harvey JB, et al. Bariatric surgery implementation trends in the USA from 2002 to 2012. Implement Sci. 2016; 11:21. Doi: 1186/s13012-016-0382-x.
- Ahmed AR, Rickards G, Johnson J, et al. Radiological findings in symptomatic internal hernias after laparoscopic gastric bypass. Obes Surg. 2009; 19(11): 1530-1535.
- Higa KD, Ho T, Boon KB. Internal hernias after laparoscopic Rouc-en-Y gastric bypass: incidence, treatment and prevention. Obes Surg. 2003; 13(3): 350-354.
- Ahmed AR, Rickards G, Johnson J, et al. Trends in internal hernia incidence after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007; 17(12): 1563-1566.
- Karila-Cohen P, Cuccioli F, Tammaro P, et al. Contribution of computed tomographic imaging to the management of acute abdominal pain after gastric bypass: correlation between radiological and surgical findings. Obes Surg. 2017; 27(8): 1961-1972.
- Karamanalos SN, Vagenas K, Kalfarentzos, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008; 247(3): 401-407.
- Scheirey CD, Scholz FJ, Shah PC, et al. Radiology of the laparoscopic roux-en-Y gastric bypass procedure: conceptualization and precise interpretation of results. Radiographics. 2006; 26(5): 1355-1371.
- Merkle EM, Hallowell PT, Crouse C, et al. Roux-en-Y gastric bypass for clinically severe obesity: normal appearance and spectrum of complications at imaging. Radiology. 2005; 234(3): 674-683.
- Fridman A, Moon R, Cozacov Y, et al. Procedure-related morbidity in bariatric surgery: A retrospective short- and mid-term follow-up of a single institution of the American College of Surgeons Bariatric Surgery Centers of Excellence. J Am Coll Surg. 2013; 217(4): 614-620.
- Carucci LR, Turner MA, Conklin RC, et al. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology. 2006; 238(1): 119-127.
- Carucci LR, Turner MA, Shaylor SD. Internal hernia following Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of radiographic findings at small-bowel examination. Radiology. 2009; 251(3): 762-770.
- Doishita S, Takeshita T, Uchima Y, et al. Internal hernias in the era of multidetector CT: correlation of imaging and surgical findings. Radiographics. 2016;36(1):88-106.
- Ximenes MAS, Baroni RH, Trindade RM, et al. Petersen’s hernia as a complication of bariatric surgery: CT findings. Abdom Imaging. 2011;36(2):126-129.
- Blachar A, Federle MP, Brancatelli G, et al. Radiologist performance in the diagnosis of internal hernia by using specific CT findings with emphasis on transmesenteric hernia. Radiology. 2001; 221(2):422-428.
Citation
DH S.Imaging of postoperative internal hernias. Appl Radiol. 2018; (11):13-18.
November 8, 2018