Imaging of intracranial infectious diseases in adults
Images



Infectious diseases affecting the central nervous system (CNS) range from acute to chronic, from indolent to life-threatening, and from self-limited to relentlessly progressive. Prompt detection of intracranial infections can have a significant effect on patient outcomes with regard to morbidity and mortality. Accurate characterization with medical imaging can allow appropriate empiric therapy to be initiated, complications to be managed appropriately and prognosis to be offered. In some cases, a specific infectious pathogen can be suggested based on typical imaging findings and clinical scenarios; other times, the broad category of infection can only be offered among a range of possible etiologies. In this article, the authors present an overview of intracranial infections, organized by category of pathogen, with an emphasis on the degree of specificity afforded by various imaging findings.1
Bacterial infections
The various typical bacteria infecting the CNS usually cannot be distinguished by imaging; rather they are grouped according to site of infection, which directs therapy. Exceptions include mycobacterial and spirochetal pathogens, which are discussed separately.
Epidural infection
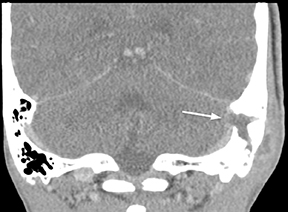
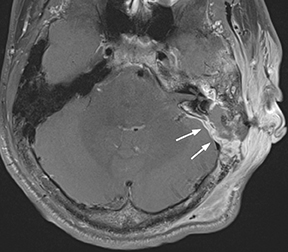
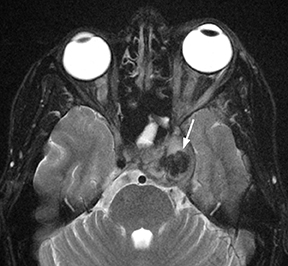
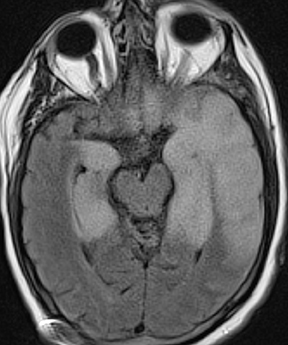
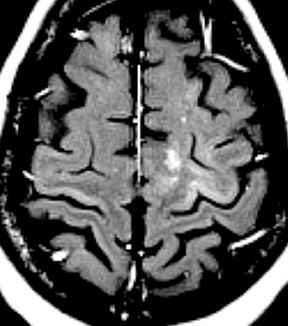
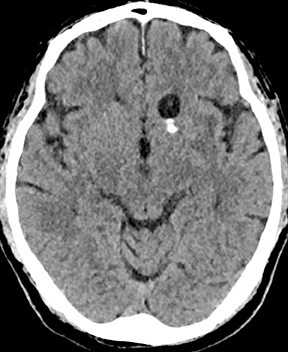
Epidural infections typically result from direct extension of an adjacent infection (commonly mastoiditis or sinusitis) or from direct seeding following neurosurgical intervention; hematogenous seeding is possible, but less common. Epidural phlegmon appears as an ill-defined, typically enhancing collection with associated dural thickening within the epidural space on postcontrast computed tomography(CT) or magnetic resonance (MR) images (Figure 1). If adjacent to a site of infection, these findings indicate extension into the epidural space. If adjacent to a site of surgery, the findings may not be reliably differentiated from sterile reactive and postsurgical changes. Progression to epidural abscess is evidenced by a well-defined lentiform fluid collection with an enhancing rim. The internal contents often demonstrate restricted diffusion; however, this can be variable given the often indolent development and commonly subacute evolution of the suppurative contents.2,3 Again, in the postoperative period, an evolving hematoma, complicated seroma, or pseudomeningocele could have a similar appearance; the presence of restricted diffusion suggests infection, but even this finding is less sensitive and specific in the postoperative state largely due to intermixed blood products.
Subdural infection
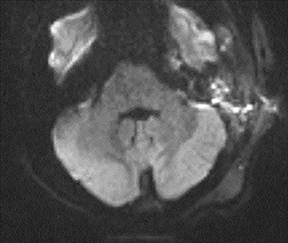
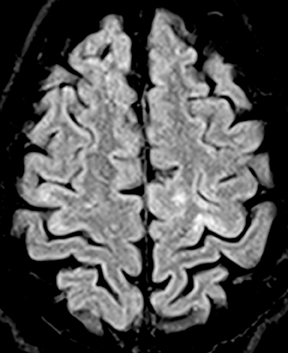
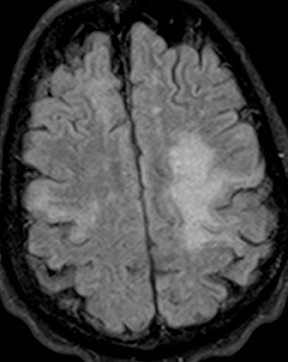
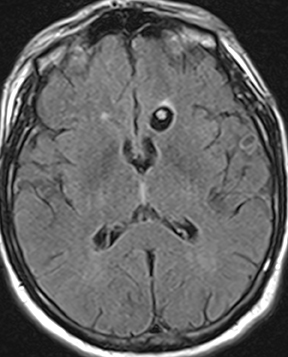
Subdural empyema refers to an infected fluid collection in the subdural space. Etiology may be related to an extradural infection (again including mastoiditis and sinusitis) that has breached the dura or may be secondary to leptomeningitis with spread to the subdural space through arachnoid villi or bridging veins; primary hematogenous seeding is also possible. The appearance on imaging is of a crescent-shaped collection with rim enhancement and internal restricted diffusion (Figure 2). In some cases, evolving subdural hematoma can have asimilar appearance, though again the combination of restricted diffusion and thick rim enhancement strongly suggests infection in the appropriate setting.2-4 As with epidural abscess, diffusion restriction is less sensitive and specific in the postoperative period.
Thrombophlebitis
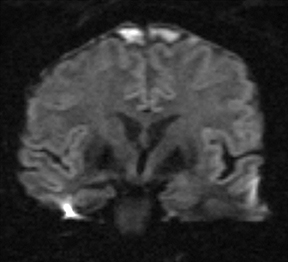
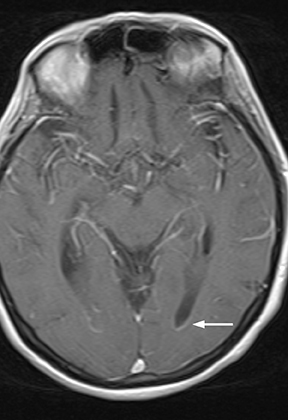
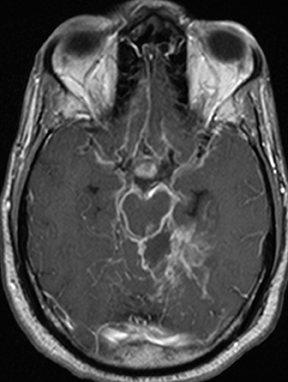
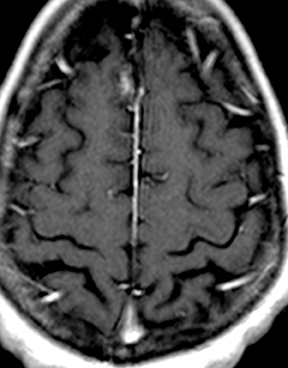
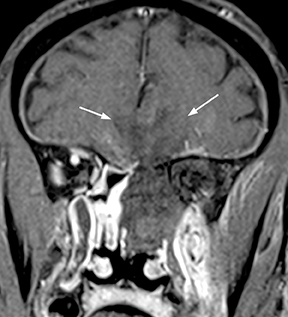
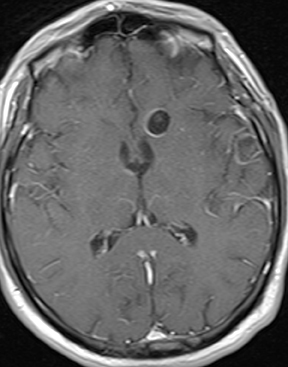
As a complication of either epidural or subdural infection, the dural venous sinuses may become involved, resulting in thrombosis with the possibility of resultant venous hypertensive edema or hemorrhage. The cavernous sinus may similarly be involved, and the cavernous segment of the internal carotid artery (ICA) may be affected with resultant vasospasm, occlusion, and/or mycotic pseudoaneurysm. Thrombophlebitis appears as a change in signal and loss of flow-void of the venous structure on spin echo MRI, often with expansion (Figure 3).5 Contrast-enhanced MR venography is highly sensitive and specific for thrombosis.6 Vascular imaging (eg, MR angiography) should be considered in cases of cavernous sinus involvement and may show narrowing or occlusion of the artery or a saccular pseudoaneurysm.7
Meningitis
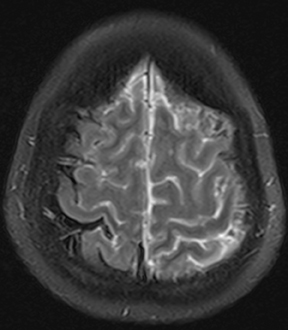
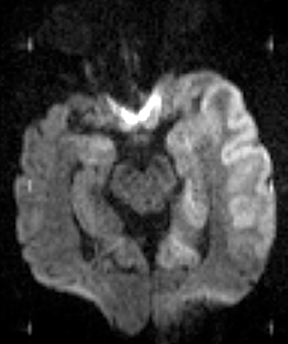
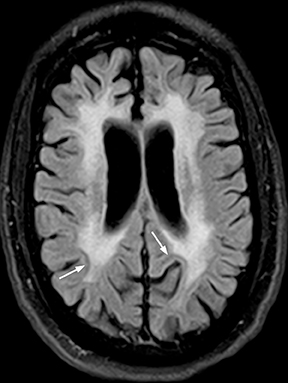
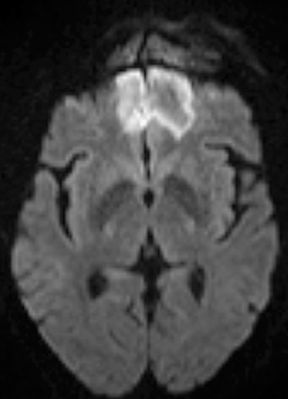
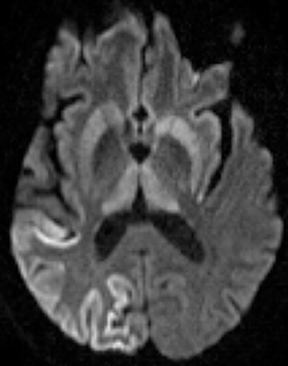
Bacterial meningitis is primarily a clinical and laboratory diagnosis. Imaging may be employed to exclude other diseases when the diagnosis is unclear or to assess for complications when the patient fails to respond to empiric therapy. CT has low sensitivity for uncomplicated meningitis; increased density within the subarachnoid spaces or leptomeningeal enhancement may be seen in some cases. MRI is more sensitive than CT.8 T2-weighted fluid-attenuated inversion-recovery (FLAIR) images may demonstrate hyperintense signal within the cerebral sulci as inflammatory exudates change the inversion time of cerebrospinal fluid (CSF). Postcontrast T1-weighted imaging may be more sensitive, demonstrating leptomeningeal enhancement.9 Recently, post-contrast T2 FLAIR images have been shown to have even higher sensitivity, demonstrating both leptomeningeal enhancement and seepage of gadolinium contrast material into the CSF (Figure 4).10
Pre- and postcontrast magnetization transfer imaging has been shown in limited investigations to provide up to 100% sensitivity for bacterial meningitis, though it remains of uncertain utility and thus not in widespread use.11
Complicated meningitis
Complications of bacterial meningitis are readily detected by CT and MR, the latter being more sensitive for certain complications. Hydrocephalus is the most common complication, caused by inflammation of arachnoid villi and decreased CSF resorption; typically the entire ventricular system is enlarged (“nonobstructive” or “communicating” hydrocephalus). Infectious processes can extend into the ventricles (ventriculitis/ependymitis), evidenced by enhancement of the ependymal lining with occasional layering purulent debris (Figure 4), and can cause adhesions and loculations, resulting in focal entrapment or obstructive hydrocephalus. Chronic hydrocephalus may result from either case, requiring long-term CSF shunting.4 Inflammation of the membranous labyrinth may occur through the cochlear aqueduct or internal auditory canal and can cause hearing loss and/or vestibular dysfunction. In the acute phase, MRI may demonstrate increased signal of labyrinthine fluid on FLAIR or enhancement of the membranous labyrinth following contrast administration. In some cases, the labyrinth may become ossified (labyrinthitis ossificans). This will be apparent on CT as increased attenuation within the cochlea, vestibule, and/or semicircular canals. MR findings include decreased signal or obliteration of fluid spaces on T2-weighted images.12 The major arteries and branches supplying the brain course in the subarachnoid spaces, and meningitis can lead to secondary arteritis. Vasospasm may be seen on angiographic studies (catheter,CT, or MR angiography), and parenchymal infarctions or septic emboli may occur. Finally, infection may extend into the subdural or epidural spaces or into the brain parenchyma.4
Parenchymal infections
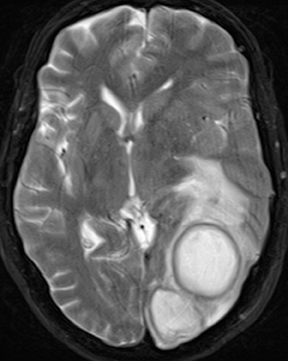
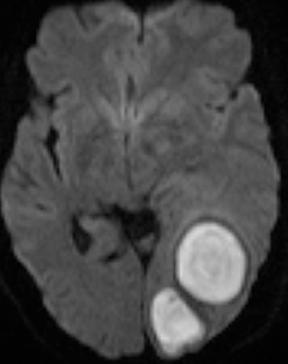
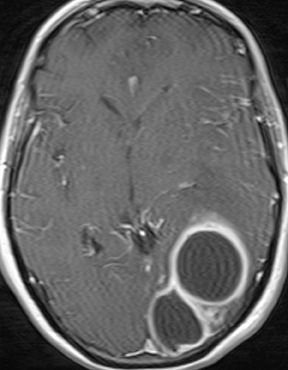
Infections of the brain parenchyma most commonly occur through hematogenous seeding, though spread from adjacent sources or seeding from trauma or surgery is also possible. Infection evolves predictably from cerebritis to abscess. Early cerebritis is characterized by vasogenic edema (low attenuation on CT; hyperintense signal on T2-weighted and FLAIR MR images) and mass effect with patchy, ill-defined enhancement. In late cerebritis, irregular rim enhancement develops. Cerebral abscess (early capsule stage) appears as a fluid-filled cavity with well-defined rim enhancement (Figure 5). The abscess capsule is typically seen on CT as higher in attenuation than the abscess core and surrounding parenchymal edema. On MRI the capsule is hypointense on T2-weighted and precontrast T1-weighted images. Avid rim enhancement following contrast administration is expected on CT or MRI. The abscess cavity contains purulent material of low attenuation on CT, hypointense signal on precontrast T1-weighted images, and hyperintense signal on T2-weighted and FLAIR images. Characteristic diffusion restriction within the cavity reliably distinguishes pyogenic abscess from toxoplasmosis, most neoplastic conditions, and other rim-enhancing lesions.13-16 MR spectroscopy will demonstrate a prominent lactate peak due to anaerobic metabolism and decreased NAA due to neuronal loss.14 Perfusion imaging will demonstrate normal to mildly decreased relative cerebral blood volume (rCBV) in the periphery and absent perfusion centrally.17 However, MR spectroscopy and perfusion are less specific than diffusion-weighted imaging and typically add little information. The final stage, late capsule, is defined by cavity collapse and decreasing parenchymal edema. Septic embolization is a special case of hematogenous dissemination occurring typically in the setting of valvular vegetation in bacterial endocarditis. Infected small particles embolize to small intracranial arteries and produce a combination of infarction and parenchymal infection. The finding of enhancement in what appears otherwise as an acute infarct should suggest the possibility of infection. Individual foci may progress to frank abscess if untreated.
CNS tuberculosis
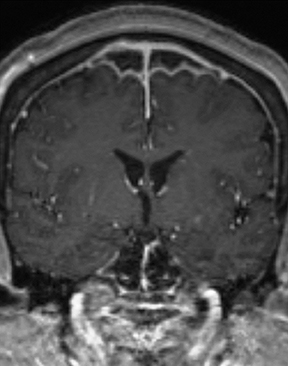
CNS infection caused by Mycobacterium tuberculosis (TB) generally has a clinical presentation and imaging appearance different from that caused by typical bacteria. The diagnosis is made by CSF sampling, but imaging findings may be suggestive. The most common presentation is basal meningitis with leptomeningeal enhancement and signal hyperintensity on T2 FLAIR images within and along the basal cisterns (Figure6). The appearance is not specific for TB, as other infectious processes (eg, from fungi and other bacteria) as well as noninfectious inflammatory(eg, sarcoidosis) and neoplastic (eg, metastases) processes can have similar features; findings of tuberculosis in other organs (most commonly the lungs) increase specificity. Complications of TB meningitis are similar to those of bacterial meningitis, most commonly including hydrocephalus and vasculitis with infarctions. Parenchymal involvement is seen in a minority of cases, but is more common in patients infected with the human immunodeficiency virus (HIV). Many cases co-exist with basal meningitis, with the combination of findings more specific for TB than either alone. The most common parenchymal finding is a tuberculoma (tuberculous granuloma). Tuberculoma can be T2-hyperintense with solid enhancement (non-caseating granuloma), T2-hypointense/isointense with rim enhancement (solid caseating granuloma), or T2-hyperintense with rim enhancement (liquid caseating granuloma). The latter appearance may also represent a true tuberculous abscess, the two being indistinguishable by imaging. Restricted diffusion is not typically seen, differentiating TB from pyogenic abscess. Spectroscopy may demonstrate an elevated lactate peak and decreased NAA. Other less common findings in CNS TB are focal cerebritis (appearing similar to bacterial cerebritis) and leukoencephalopathy (appearing as a diffuse demyelinating process). Treated lesions often become calcified.18,19
Neurosyphilis
Like tuberculosis, neurosyphilis tends to involve the basal meninges. Vasculitis and infarctions are commonly seen. Parenchymal involvement may also be seen and manifest as nonenhancing white matter lesions or, rarely, enhancing cerebral gummas. Cerebral atrophy may be seen in the chronic phase in patients with dementia. In the current age, the majority of patients with neurosyphilis are also HIV-positive (largely dueto shared risk factors), therefore, intracranial manifestations of HIV/AIDS and opportunistic infections may co-exist with neurosyphilis, potentially confounding the imaging findings.20,21
Lyme disease
Intracranial manifestations of Lyme disease (Lyme neuroborreliosis) assume two primary forms which, when seen together, allow this disease to be suggested with relative specificity. The first includes parenchymal lesions (low attenuation on CT and hyperintense on FLAIR with variable enhancement) affecting the subcortical (primarily frontal) and periventricular white matter in a pattern that mimics multiple sclerosis or other demyelinating diseases. The second pattern is a cranial neuritis—enlargement and enhancement of cranial nerves; the facial nerves are most commonly affected (rendering Lyme disease a differential consideration in cases of Bell’s palsy), with oculomotor and trigeminal nerve involvement also reported.21,22
Viral infections
Viral infections of the CNS typically manifest as meningitis or encephalitis (or combined meningoencephalitis). A few pathogens with
characteristic imaging features are highlighted here.
Herpes simplex virus (HSV)
Herpes simplex virus (HSV) is notable for its typical imaging appearance and because it is readily treatable when diagnosed early, but potentially devastating in cases of delayed diagnosis. The typical appearance of HSV encephalitis includes parenchymal edema (hypoattenuating onCT; hyperintense on FLAIR) with variable patchy or gyriform enhancement and variable degrees of restricted diffusion (Figure 7); parenchymal hemorrhage and necrosis may occur in severe or untreated cases. MRI findings are seen earlier in the course of infection than CT findings, withDWI changes most sensitive among routine sequences. The classic distribution includes bilateral (though asymmetric) involvement of the limbic system (most notably the mesial temporal lobes). Other cortical regions may also be involved, while the basal ganglia are typically spared.23,24
Cytomegalovirus (CMV)
Cytomegalovirus (CMV) meningoencephalitis is typically seen in immunocompromised patients. Ventriculitis is a classic feature, seen as ependymal enhancement, ventriculomegaly, and occasionally layering debris. CMV encephalitis has a predilection for the periventricular/subependymal regions. Parenchymal lesions may be mass-like, but tend not to enhance except in the late necrotic stage.23,24
Human immunodeficiency virus (HIV)
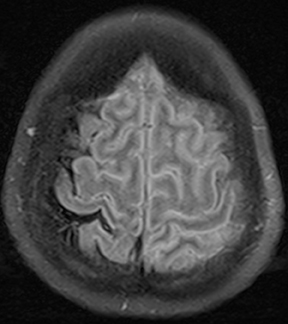
Human immunodeficiency virus (HIV), besides opening the door for opportunistic infections, can infect the CNS directly. In fact, HIV encephalopathy is the most common neurologic manifestation of HIV infection. This encompasses both an acute meningoencephalitis sometimes seen at the time of seroconversion and a spectrum of chronic diseases known as HIV-associated neurocognitive disorder(HAND). The latter is typically progressive and includes HIV-associated dementia (formerly AIDS dementia complex) as its most severe form. Imaging features tend to progress with the disease, but do not strictly correlate with clinical severity. These include cerebral atrophy and symmetric, typically confluent periventricular and deep white matter lesions without enhancement (Figure 8).24,25
Progressive multifocal leukoencephalopathy (PML)
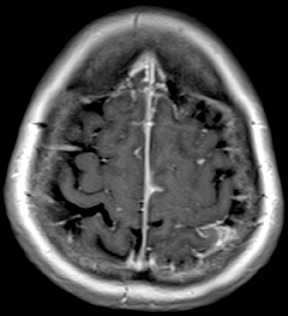
Progressive multifocal leukoencephalopathy (PML) is an opportunistic infection caused by the JC virus and seen almost exclusively in immuno-compromised patients. The typical appearance includes asymmetric white matter lesions, predominantly subcortical with characteristic involvement of U-fibers, without mass effect or enhancement (Figure 9). Gray matter structures may also be involved. Longstanding infections may begin to show faint peripheral enhancement. If a patient with AIDS and PML is started on anti-retroviral therapy, the lesions may begin to enhance, and symptoms may worsen temporarily, a condition known as immune reconstitution inflammatory syndrome (IRIS).24,26,27
Other viral infections
Varicella zoster virus (VZV) can cause acute meningitis, notable for its propensity to cause arteritis and infarcts, or reactivation infection of the facial nerve (herpes zoster oticus; Ramsay Hunt syndrome), appearing as abnormal facial nerve enhancement.23 A group of arthropod borne viruses (arboviruses, of which West Nile virus is an example) can cause encephalitis with patchy parenchymal signal changes and variable enhancement; involvement of the deep gray nuclei is common. Cerebellitis and rhombencephalitis are terms referring to encephalitis of the cerebellum and/or brain stem, typically caused by a variety of viral pathogens. A host of other viral pathogens may have variable nonspecific imaging findings.24
Fungal infections
Invasive fungal sinusitis
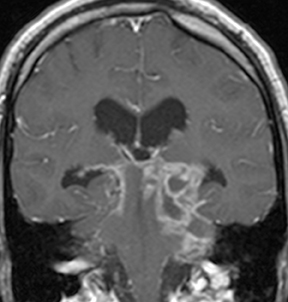
Seen primarily in neutropenic and diabetic patients, invasive fungal sinusitis is caused by Aspergillus or Zygomycetes (typically Mucorales) species and leads to rapid involvement of the orbits, cavernous sinus, and intracranial structures with a high risk of death or serious disability. Variable amounts of sinus disease may be seen, but extension into the soft tissues and orbits differentiates this serious disease from more benign forms of sinusitis. Epidural phlegmon and leptomeningeal enhancement are indicators of intracranial spread.Direct parenchymal infection or infarcts from arterial invasion are the most severe complications (Figure 10).28
Cryptococcus
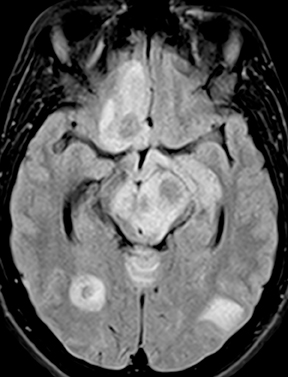
Cryptococcal meningitis is seen most commonly in immunocompromised patients. Nonspecific leptomeningeal enhancement may be seen. The fungi may produce a mucinous material that fills and dilates perivascular spaces within the deep-gray and white-matter structures; these are known as gelatinous pseudocysts and tend to mirror CSF in attenuation and signal characteristics without enhancement or diffusion restriction (Figure 11). Rarely, parenchymal involvement may occur, either with one or more rim-enhancing or solidly enhancing masses (cryptococcomas) or with a miliary pattern of small enhancing nodules.28 Rare reports in immunocompetent hosts have underscored the potential for atypical imaging appearances in such cases, including robust enhancement, diffusion restriction, and inflammatory changes.29
Other fungal infections
Coccidiomycosis, histoplasmosis, and other fungal infections may involve the meninges and/or parenchyma to varying degrees and maybe more common and more severe in immunocompromised patients. Basal meningitis is an especially prominent feature in many fungal infections.28
Parasitic infections
Toxoplasmosis
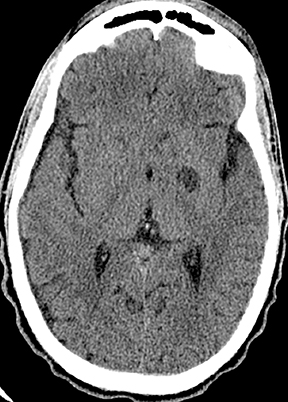
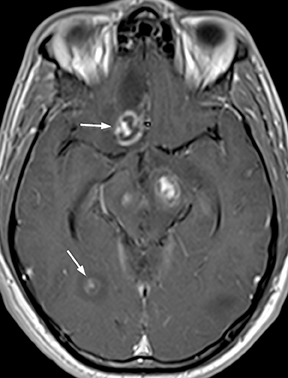
CNS toxoplasmosis is among the most common CNS manifestations of AIDS.21 Parenchymal lesions are typically multiple (though occasionally solitary) and most often located in the deep gray nuclei or at the gray-white junctions in the cerebral hemispheres. Larger lesions are rim enhancing, sometimes with a central dot creating a classic “target” appearance; surrounding parenchymal edema and mass effect are variable,while increased diffusivity (rather than restricted diffusion) of the T2-hyperintense center differentiates these from pyogenic abscesses (Figure12).13 Small lesions may demonstrate nodular enhancement. When differentiation from CNS lymphoma is necessary, thallium-201 scintigraphy will be negative for toxoplasma lesions, while lymphoma reliably demonstrates intense uptake. Further specificity may sometimes be provided by relative apparent diffusion coefficient (ADC) values, tending towards greater ratios versus normal white matter in toxoplasmosis. Proton MR spectroscopy may occasionally function to differentiate the two processes, demonstrating elevations in the lipid-lactate resonance frequencies in toxoplasmosis, without the elevations in choline resonance sometimes encountered in CNS lymphoma. Perfusion imaging in toxoplasmosis does not commonly demonstrate the perfusion elevations characteristic of lymphoma. Notwithstanding the above considerations, it is the authors’ opinion that imaging features alone are commonly insufficient to conclusively narrow the differential diagnosis between these entities, and therapeutic response monitoring remains common. Treated lesions may develop calcification.
Cysticercosis
Neurocysticercosis is characterized by lesions exhibiting characteristic evolution through stages with unique imaging features.30 Lesions maybe parenchymal (though often on the surface), subarachnoid/cisternal, or intraventricular. The first stage (vesicular) is cystic in nature, with internal fluid of CSF attenuation and signal characteristics, sometimes with thin rim enhancement, and without significant parenchymaledema. An intracystic nodule (best seen as hyperintense to fluid on FLAIR or DWI) representing the scolex (head of the tapeworm) may be seen (Figure 13). In the second stage (colloid vesicular), the larva degenerates, the fluid becomes complex (increased attenuation on CT;increased signal on FLAIR; occasional diffusion restriction31), perilesional edema develops, the cyst wall thickens and enhances, and the scolex enhances. Finding a lesion with a cyst and scolex (vesicular or colloid vesicular stage) is sufficient to diagnose neurocysticercosis according to accepted diagnostic criteria.32 In the third stage (granular nodular), the cyst retracts and edema decreases, leaving only an enhancing nodule. Once healing is complete, a nonenhancing calcified nodule remains and may persist indefinitely (nodular calcified stage);this is best seen on CT, while GRE and SWI are most sensitive among widely available MR sequences. In a given patient, lesions in different stages may coexist. A “racemose” variety involves clustered cysts in the subarachnoid spaces, often without a visible scolex, and may not proceed through the typical stages.33
Other parasitic infections
Various amebic parasites can infect the CNS, often causing a severe and relentless meningoencephalitis. Imaging findings are varied and nonspecific, typically including enhancing meninges and/or parenchymal mass lesions.21
Prion disease
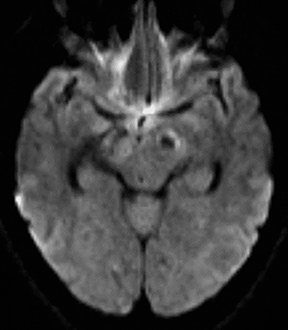
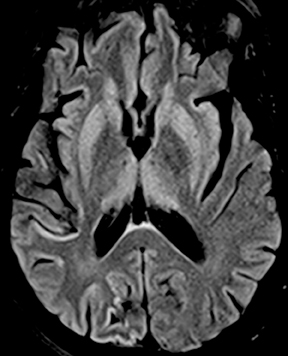
While often categorized as infectious because they can be transmitted from one host to another, prions are not microorganisms but rather native proteins folded in an abnormal configuration. By far the most common prion disease in humans is Creutzfeldt-Jakob disease (CJD),clinically manifested as a rapidly progressive dementia and myoclonus. Imaging findings are rather specific in the appropriate clinical setting, often preceding the full clinical syndrome or characteristic electroencephalographic findings. Findings include nonenhancing signal abnormalities involving the supratentorial gray matter structures. Increased signal on DWI typically precedes or exceeds that seen onFLAIR (Figure 14). The cerebral cortices, caudate nuclei, and putamina are commonly involved in sporadic CJD, which is typically bilateral though perhaps asymmetric. Involvement of the dorsal thalamus (“pulvinar sign”) and medial and posterior thalamus (“hockey stick sign”)were originally described as being specific for variant CJD but can be seen in the more common sporadic form as well.34 It has been suggested that defining the “pulvinar sign” as signal intensity (on FLAIR images) in the pulvinar greater than that in the anterior putamen affords a high degree of specificity for variant CJD35, though this has not yet been fully validated.
Conclusion
This article describes typical features of various intracranial infectious diseases. While some cases may be pathognomonic in imaging appearance, most will require a combination of clinical, laboratory, and imaging data to arrive at a diagnosis.
References
- Hygino da Cruz LCJ, Domingues RC. Intracranial infections. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. Vol 1. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:929-1025.
- Karampekios S, Hesselink J. Cerebral infections. Eur Radiol. 2005;15:485-493.
- Tsuchiya K, Osawa A, Katase S, et al. Diffusion-weighted MRI of subdural and epidural empyemas. Neuroradiology. 2003;45:220-223.
- Mohan S, Jain KK, Arabi M, Shah GV. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am. 2012;22:557-583.
- Schuknecht B, Simmen D, Yuksel C, Valavanis A. Tributary venosinus occlusion and septic cavernous sinus thrombosis: CT and MR findings. AJNR Am J Neuroradiol. 1998;19:617-626.
- Kirchhof K, Welzel T, Jansen O, Sartor K. More reliable noninvasive visualization of the cerebral veins and dural sinuses: Comparison of three MR angiographic techniques. Radiology. 2002;224:804-810.
- Cloud GC, Rich PM, Markus HS. Serial MRI of a mycotic aneurysm of the cavernous carotid artery. Neuroradiology. 2003;45:546-549.
- Chang KH, Han MH, Roh JK, et al. Gd-DTPA-enhanced MR imaging of the brain in patients with meningitis: Comparison with CT. AJR Am J Roentgenol. 1990;154:809-816.
- Kamran S, Bener AB, Alper D, Bakshi R. Role of fluid-attenuated inversion recovery in the diagnosis of meningitis: Comparison with contrast-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2004;28:68-72.
- Splendiani A, Puglielli E, De Amicis R, et al. Contrast-enhanced FLAIR in the early diagnosis of infectious meningitis. Neuroradiology. 2005;47:591-598.
- Kamra P, Azad R, Prasad KN, et al. Infectious meningitis: Prospective evaluation with magnetization transfer MRI. Br J Radiol. 2004;77:387-394.
- Casselman JW, Mark AS, Butman JA. Anatomy and Diseases of the Temporal Bone. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. Vol 2. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:1193-1257.
- Chong-Han CH, Cortez SC, Tung GA. Diffusion-weighted MRI of cerebral toxoplasma abscess. AJR Am J Roentgenol. 2003;181:1711-1714.
- Lai PH, Hsu SS, Ding SW, et al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging in intracranial cystic mass lesions. Surg Neurol. 2007;68 Suppl 1:S25-36.
- Bukte Y, Paksoy Y, Genc E, Uca AU. Role of diffusion-weighted MR in differential diagnosis of intracranial cystic lesions. Clin Radiol. 2005;60:375-383.
- Fertikh D, Krejza J, Cunqueiro A, et al. Discrimination of capsular stage brain abscesses from necrotic or cystic neoplasms using diffusion-weighted magnetic resonance imaging. J Neurosurg. 2007;106:76-81.
- Muccio CF, Esposito G, Bartolini A, Cerase A. Cerebral abscesses and necrotic cerebral tumours: Differential diagnosis by perfusion-weighted magnetic resonance imaging. Radiol Med. 2008;113:747-757.
- Bernaerts A, Vanhoenacker FM, Parizel PM, et al. Tuberculosis of the central nervous system: Overview of neuroradiological findings. Eur Radiol. 2003;13:1876-1890.
- Patkar D, Narang J, Yanamandala R, et al. Central nervous system tuberculosis: Pathophysiology and imaging findings. Neuroimaging Clin N Am. 2012;22:677-705.
- Brightbill TC, Ihmeidan IH, Post MJ, et al. Neurosyphilis in HIV-positive and HIV-negativepatients: Neuroimaging findings. AJNR Am J Neuroradiol. 1995;16:703-711.
- Akgoz A, Mukundan S, Lee TC. Imaging of rickettsial, spirochetal, and parasitic infections. Neuroimaging Clin N Am. 2012;22:633-657.
- Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme neuroborreliosis: Manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol. 2009;30:1079-1087.
- Bulakbasi N, Kocaoglu M. Central nervous system infections of herpesvirus family. Neuroimaging Clin N Am. 2008;18:53-84; viii.
- Solbrig MV, Hasso AN, Jay CA. CNS viruses--diagnostic approach. Neuroimaging Clin N Am. 2008;18:1-18; vii.
- Post MJ, Tate LG, Quencer RM, et al. CT, MR, and pathology in HIV encephalitis and meningitis. AJR Am J Roentgenol. 1988;151:373-380.
- Whiteman ML, Post MJ, Berger JR, et al. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: Neuroimaging with clinical and pathologic correlation. Radiology. 1993;187:233-240.
- Rajeswaran G, Becker JL, Michailidis C, et al. The radiology of IRIS (immune reconstitution inflammatory syndrome) in patients with mycobacterial tuberculosis and HIV co-infection: Appearances in 11 patients. Clin Radiol. 2006;61: 833-843.
- Mathur M, Johnson CE, Sze G. Fungal infections of the central nervous system. Neuroimaging Clin N Am. 2012;22:609-632.
- Saigal G, Post MJ, Lolayekar S, Murtaza A. Unusual presentation of central nervous system cryptococcal infection in an immunocompetent patient. AJNR Am J Neuroradiol. 2005;26:2522-2526.
- Lucato LT, Guedes MS, Sato JR, et al. The role of conventional MR imaging sequences in the evaluation of neurocysticercosis: Impact on characterization of the scolex and lesion burden. AJNR Am J Neuroradiol. 2007;28:1501-1504.
- Santos GT, Leite CC, Machado LR, et al. Reduced diffusion in neurocysticercosis: Circumstances of appearance and possible natural history implications. AJNR Am J Neuroradiol. 19 2012 [Epub ahead of print].
- Del Brutto OH, Rajshekhar V, White AC, Jr., et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 24 2001;57:177-183.
- Lerner A, Shiroishi MS, Zee CS, et al. Imaging of neurocysticercosis. Neuroimaging Clin N Am. 2012;22:659-676.
- Kallenberg K, Schulz-Schaeffer WJ, Jastrow U, et al. Creutzfeldt-Jakob disease: Comparative analysis of MR imaging sequences. AJNR Am J Neuroradiol. 2006;27:14591462.
- Collie DA, Summers DM, Sellar RJ, et al. Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am J Neuroradiol. 2003;24:1560-1569.
Related Articles
Citation
Imaging of intracranial infectious diseases in adults. Appl Radiol.
February 6, 2014