FDG-PET Predicts Neoadjuvant Therapy Response in Pre-Op Pancreatic Cancer Patients
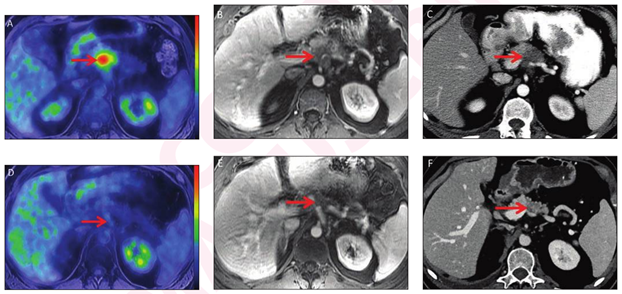 A study published in the Journal of the National Comprehensive Cancer Network reports that FDG-PET was superior to biochemical responses alone in predicting pathologic response and survival among patients with post neoadjuvant therapy (NAT) borderline resectable/locally advanced (BR/LA) pancreatic ductal adenocarcinoma (PDAC). As a pre-operative metric of NAT efficacy, FDG-PET may allow for therapeutic alterations and surgical treatment decisions.
A study published in the Journal of the National Comprehensive Cancer Network reports that FDG-PET was superior to biochemical responses alone in predicting pathologic response and survival among patients with post neoadjuvant therapy (NAT) borderline resectable/locally advanced (BR/LA) pancreatic ductal adenocarcinoma (PDAC). As a pre-operative metric of NAT efficacy, FDG-PET may allow for therapeutic alterations and surgical treatment decisions.
The Mayo Clinic study included 202 patients who underwent at least one FDG-PET post-NAT within 60days of resection. Of these patients, 58% had optimization of biochemical (CA 19-9) levels, 51% had major metabolic responses and 38% had pathologic responses. Median times to recurrence-free survival (RFS) and overall survival (OS) were 21 and 48.7 months, respectively.
Metabolic response was found to be superior to biochemical response in predicting pathologic response and it was the only univariate preoperative predictor of OS and highly correlated with pathologic response.
At follow-up, 41% of the patients developed recurrence and 69% remained alive. The authors report that “ Biochemical response in the absence of an associated major metabolic response did not correlate with a subsequent major pathologic response (major pathologic response among patients with optimal CA 19-9 level and minor metabolic response, 7.1%; among patients with major CA 19-9 response and minor metabolic response, 3.8%), suggesting that CA 19-9 alone is insufficient in determining the adequacy of NAT response.”
The authors conclude their results of the study may be sufficient to utilize FDG-PET as an adjunct modality in assessing NAT efficacy in addition to the currently approved but poorly predictive metrics (CT/MRI and CA 19-9 level) and use this information with “all available response measures (i.e., clinical, radiologic, biochemical, and metabolic) to make suitable decisions regarding NAT alterations and final decisions for surgery or no surgery on a case-by-case basis.”
Related Articles
Citation
FDG-PET Predicts Neoadjuvant Therapy Response in Pre-Op Pancreatic Cancer Patients. Appl Radiol.
October 7, 2022