FDG-PET Predicts Chemotherapy Response in Pancreatic Patients Prior to Surgery
 Research in the Journal of the National Comprehensive Cancer Network reports the use of positron emission tomography (PET) with 18-fluorodeoxyglucose (FDG) tracer adds significant prognostic benefit in objectively assessing neoadjuvant chemotherapy response in borderline resectable/locally advanced pancreatic cancer patients prior to surgery.
Research in the Journal of the National Comprehensive Cancer Network reports the use of positron emission tomography (PET) with 18-fluorodeoxyglucose (FDG) tracer adds significant prognostic benefit in objectively assessing neoadjuvant chemotherapy response in borderline resectable/locally advanced pancreatic cancer patients prior to surgery.
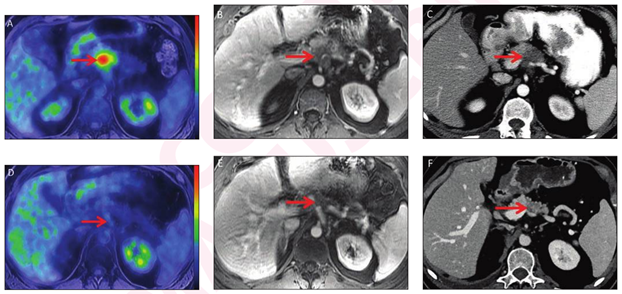
"We were astonished by how metabolic imaging can now predict outcomes with high accuracy before any surgical intervention,” said senior research Mark J. Truty, MD, MS, Mayo Clinic Comprehensive Cancer Center. “Not only that, but FDG-PET response was the single largest preoperative predictor of survival for these patients. FDG-PET decisively identified more than 85% of the patients suspected to have major pathological responses. This is a very impressive proportion and better than the currently-available biochemical response assessment through CA 19-9 levels alone, and far superior to standard imaging, which is not at all predictive."
"Because we intend to use preoperative chemotherapy to benefit patients with pancreatic cancer, we need to be sure that therapy is doing what we think it's doing—killing the tumor," agreed co-lead author Ajit H Goenka, MD, also with the Mayo Clinic. “We must ‘do no harm’ by objectively showing treatment efficacy before complex surgical resection. That is what the FDG-PET scan allows—to see whether tumor is still viable or not after initial treatment, in order to help us make significant treatment decisions to proceed with complex surgery, continue current treatment, or consider a chemotherapy switch."
According to previous studies, traditional imaging modalities such as CT and/or MRI are ineffective at predicting outcomes from the pre-surgery treatment of neoadjuvant chemotherapy in pancreatic cancer patients. Measuring biochemical CA 19-9 level changes is similarly inconsistent and not possible in a significant proportion of patients. This left clinicians without many options for assessing the likelihood of long-term survival before initiating major surgery.
“Previously, we needed to wait until after complex surgery to tell how the pancreatic cancer responded to the neoadjuvant therapy,” said lead researcher Amro M Abdelrahman, MBBS, MS, Mayo Clinic. “Now that is not the case. With FDG-PET we can tell patients how the cancer responded to neoadjuvant therapy before going through major surgical resection. Going forward, we encourage providers to combine all available response measures (i.e. clinical, radiologic, biochemical, and metabolic) to make suitable decisions about neoadjuvant therapy alterations and final decisions for surgery or no surgery on a case-by-case basis."
The study included 202 patients with borderline resectable/locally advanced pancreatic cancer who received either mFOLFIRINOX or gemcitabine/nab-paclitaxel as first-line neoadjuvant chemotherapy. Major metabolic response captured by FDG-PET was highly associated with major pathologic response, i.e. tumor reduction, regardless of biochemical response as measured by CA 19-9 levels. Both factors combined were even more predictive.
“Given the very aggressive nature of pancreatic cancer, knowing if a pancreatic tumor has good response to the pre-operative treatment indicating a favorable outcome and better survival after surgery—versus no response or only partial response, indicating more aggressive tumors that may necessitate additional or alternate preoperative therapy—has been particularly challenging for clinicians for a long time,” commented Mahmoud M Al-Hawary, MD, a radiologist at University of Michigan Rogel Cancer Center, who was not involved with this research. “CT and MRI, which are the standard of care for staging pancreatic cancer at presentation before the start of chemotherapy, have limited ability to distinguish viable tumor from scar tissue, since both look similar. Even when tumors are responding, it can be difficult to assess how much via standard imaging alone.”
Dr Al-Hawary, who is a member of the NCCN Guidelines® Panel for Pancreatic Adenocarcinoma, continued, “To answer this question, we need a different type of imaging, one not based on size or shape but some other indicator of tumor function and viability to improve upon the limited clinical markers that are in current use. PET imaging can provide this functional information by showing presence or absence of tumor activity, which has been extensively proven to predict tumor response in various solid tumors. This study suggests PET can demonstrate the same in pancreatic cancer, to help stratify patients and guide treatment before they go to surgery, in conjunction with the existing blood and standard imaging indicators. Further study in wider patient groups in different institutions will help confirm this value and could potentially change practice patterns.”
Related Articles
Citation
FDG-PET Predicts Chemotherapy Response in Pancreatic Patients Prior to Surgery. Appl Radiol.
September 8, 2022