FDA clears premium digital radiography system from Philips
Images
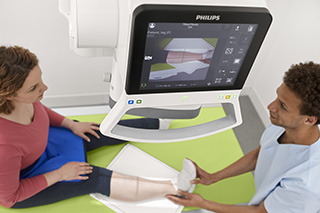
The U.S. Food and Drug Administration has given 510(k) clearance to Royal Philips to sell its newest premium digital radiography system, the DigitalDiagnost C90, in the United States. The system is designed to increase patient throughput and decrease the time to diagnose.
 The system is the first radiography unit with a live camera image directly displayed at the tube’s head, according to Philips Healthcare. This provides radiologic technologists with a clear view of the anatomical area being scanned as a patient is being positioned.
The system is the first radiography unit with a live camera image directly displayed at the tube’s head, according to Philips Healthcare. This provides radiologic technologists with a clear view of the anatomical area being scanned as a patient is being positioned.
The DigitalDiagnost C90 incorporates Philips’ UNIQUE 2 image processing and bone suppression software. It also incorporates the Philips Eleva user interface, a common platform across a range of Philips digital radiography systems that enables a smooth and efficient patient-focused workflow. This common user interface is now extended to the Eleva Tube Head, potentially speeding up workflow by over 17% per examination. Its touch screen display transfers operation into the examination room to allow for more time with the patient. DigitalDiagnost is also designed to help contribute to a lower cost of care, with flexible room configuration options and SkyPlate sharing available among all Philips premium digital radiography systems.
Citation
. FDA clears premium digital radiography system from Philips. Appl Radiol.
March 8, 2019