Duplex ultrasound for superficial venous insufficiency: Use for diagnosis and guiding therapy
Images

Dr. Khilnani is the Director of Fibroid Treatment and the Co-Director of Vein Treatment and Dr. Min is the Director of Vein Treatment, Cornell Vascular, Weill Medical College of Cornell University, New York, NY.
Recent advances in the diagnosis and treatment of superficial venous insufficiency (SVI) have provided new enthusiasm for the care of this prevalent medical condition. Many of the advances resulted from the liberal use of duplex ultrasound (DUS), which fostered a greater understanding of the anatomy and pathophysiology of this disease. In addition, the use of DUS has, in part, led to the development of safe and effective image-guided endovenous methods to ablate incompetent truncal veins. In this presentation, we will review the techniques of ultrasound (US) evaluation of the superficial venous system and demonstrate its utility in guiding and evaluating the results of endovenous ablation.
Indications for US evaluation
All patients with varicose veins require an ultrasound of the superficial venous system to map the pattern of venous incompetence prior to making treatment recommendations. Even though many patients present with varicose vein patterns that are very suggestive of a certain pathophysiology, the patterns of varicose veins seen on physical examination associated with different forms of venous incompetence overlap sufficiently to commonly result in errors of treatment selection. Patients with spider veins, particularly those in the lateral thigh area, do not routinely require US evaluation. However, when the spider veins are found in the distribution of a large truncal vein, such as the great saphenous vein (GSV), it is recommended that DUS be performed. If truncal vein refiux is identified and is contributory in these cases, treatment of this refiux may be warranted prior to treatment of the spider veins.
Diagnostic duplex US technique and findings
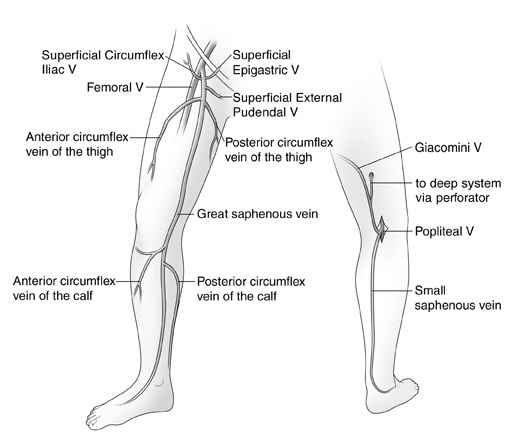
The duplex examination to evaluate for SVI has been well described. 1 Typically, a DUS unit with a 7.5 -MHz probe is used. The goals of the examination are to define the incompetent veins and to determine if they are responsible for the patient's clinical problem. When evaluating patients for refiux, the examination should be performed in the physiologically appropriate standing position. The patient is usually positioned on a stand to elevate the legs to facilitate the performance of the study. The patient is asked to turn his/her leg outward to facilitate scanning of the inner thigh and calf. Generally, the examination begins at the saphenofemoral junction (SFJ). The femoral vein is evaluated for obstruction and refiux. Next, the GSV is followed from its junction down toward the ankle. The relationship of the GSV and its tributaries to any abnormal veins is assessed by tracing their course. It is important to be aware of the standard tributary anatomy of the GSV and to recognize the frequent variations that are found 1,2 (Figure 1). One particularly common variant is the anterior accessory GSV (AAGSV), which courses anterior to the path of the GSV. It has been described in the past as a long anterior circumfiex vein or as a duplicated GSV. This vein joins the GSV just below the SFJ and then courses obliquely down the anterior thigh. It is often responsible for anterior thigh varicose veins. It may rejoin the GSV in the lower thigh.
A careful search for significant incompetent perforating veins (IPVs) into the GSV should be made. Often the caliber of the GSV will increase at the level of a significant IPV, and this may be a clue to pursue careful imaging at this level to identify this important source of refiux. Incompetent perforating veins are frequently identified at the peripheral or lower end of an incompetent vein segment; these can be called reentry IPVs. Many IPVs will normalize after correction of the truncal or nontruncal refiux feeding them.
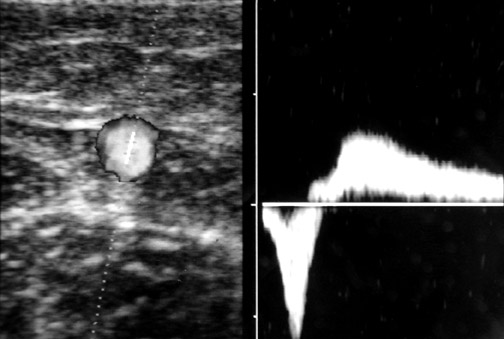
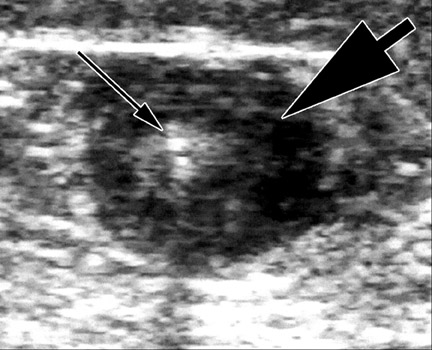
The caliber of the GSV is then assessed. Normally the vein is ≤4 mm in diameter. Veins >7 mm have a high incidence of refiux. Refiux can occur in smaller veins, but even if found, it is usually clinically unimportant. Peripheral to the takeoff of incompetent tributary veins, the caliber of the GSV often decreases. Any vein segment suspected of having refiux by size or by relationship to varicose veins is then evaluated with color and pulse wave Doppler (PWD) to directly visualize the direction of fiow. Color is very helpful to quickly exclude refiux, but can over- or underestimate its severity. All suspicious segments should be examined with PWD. Refiux can be easily documented by looking for antegrade fiow followed by retrograde fiow after a quick, firm compression of a peripheral segment of the GSV. Generally, when evaluating the GSV, compression of the calf should lead to augmen- tation of antegrade fiow. When evaluating the AAGSV, compression of the lower thigh is most useful. Upon release of compression, little if any retrograde fiow should be noted. While the literature denotes ≥0.5 seconds of retrograde fiow as the criterion for pathological refiux, several seconds of retrograde fiow is typically seen in patients with venous incompetence 1 (Figure 2).
Next, the patient is turned to face away from the examiner, and the small saphenous vein (SSV) is evaluated. The pro-cess of evaluation is similar to that of the GSV. This includes tracing the course of the SSV vein, assessing its size and its relationship to any varicose veins, and also assessing the popliteal vein. The anatomy of the SSV and its cephalic termination are quite variable. In approximately 50% to 70% of cases, the SSV terminates into the popliteal vein roughly 2 cm above the popliteal crease. However, more cephalad extension of this vein is common. Often it will extend several centimeters up the posterior thigh to terminate in a perforating vein (PV). Occasionally, it will extend even more cephalad toward the buttock crease before terminating into a PV. A relatively common variant, the vein of Giacomini, is a connection between the GSV and the SSV. This vein runs subfacially in the posterior thigh and can be a pathway of venous incompetence leading to the posterior thigh and calf varicose veins. 3
In some cases, DUS in patients with varicose veins will not identify truncal vein incompetence. These nontruncal pathways are much more common in multiparous women and include pudendal and gluteal vein incompetence 4 (Figure 1). Occasionally, these sources- especially the pudendal source-can lead to GSV incompetence more peripherally in the leg. Other important sources of nontruncal refiux include IPVs in the medial and lateral thigh and popliteal fossa that can usually be identified with DUS.
DUS in guiding sclerotherapyUltrasound-guided therapy for venous disease began more than 15 years ago with the introduction of US-guided sclero-therapy (UGS). Currently, UGS is occasionally used to direct sclerotherapy of deeper superficial veins and IPVs. For some time, there was a great deal of enthusiasm for UGS of the saphenous veins. However, high rates of recanalization reported by most investigators led to more cautious application of this technique. Recent interest in the use of foam sclero-sants for UGS of both the incompetent tributary veins and the saphenous trunks may lead to greater utilization of UGS.
UGS utilizes the principles of sclero-therapy and the advantages of image guidance. Accurate identification of in-competent vein segments and distinction from adjoining normal veins and arteries improve the success and minimize the risk associated with sclerotherapy of deeper and larger veins. Ultrasound guidance is first used to identify the target veins for therapy that often cannot be identified with the naked eye. Once selected, the vein can be punctured with real-time US guidance. This can be done with a longitudinal or transverse ap-proach. During injection, microbubbles in the sclerosant produce echoes that visibly confirm an intravascular injection. This phenomenon is more pronounced when foam sclerosants are used. After injection, successfully treated veins often go into spasm. Follow-up US several weeks after injection often reveals noncompressible veins filled with trapped blood. This usually resorbs over several months, at which point the successfully sclerosed vein becomes a cord that may be difficult to find with US.
DUS guidance of endovenous thermal ablation
Duplex ultrasound-guided endovenous thermal ablation (EVTA) is an ex-tension of the use of US to guide sclero- therapy. Laser and radiofrequency EVTA of the saphenous veins and their primary tributaries utilizes catheters inserted into the abnormal vein peripherally and ad-vanced to the most central level of refiux. These catheters are then activated (creating endovenous heat) and withdrawn across the segment to be treated. The goals of these therapies are to permanently occlude the incompetent vein segments. 5,6
Duplex ultrasound is used first to identify the location for venous access. This is generally at the lowest or most peripheral level of the primary incompetent segment. Typically, the GSV refiuxes from the SFV down toward the calf, where its incompetent fiow spills into a tributary that fills varicosities. In these cases, the access will be at the level of the takeoff of this tributary. The GSV is usually small further down the calf; the size transition seen on ultrasound guides where to access the GSV. Occasionally, the puncture and treatment can be applied to superficial tributaries that remain relatively straight and parallel to the course of the GSV. In this case, these so-called superficial accessory saphenous veins can be accessed directly at the most peripheral part of the straight segment.
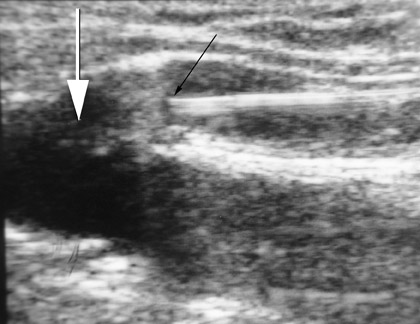
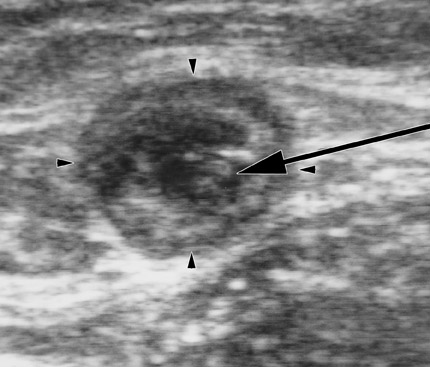
After access, DUS is used to position the catheter to the highest level to be ablated. In the case of the GSV, this is usually at the SFJ. With the laser catheter, a long sheath is usually inserted into the femoral vein and the laser fiber is placed through it. In the case of the SFJ, the fiber and sheath are withdrawn together until the tip of the fiber is at or just below the SFJ (Figure 3). This is best performed by imaging in a longitudinal projection. With the radiofrequency (RF) device, a short sheath is placed and the RF catheter inserted. The RF device can track over a wire. The device is advanced to the SFJ, and its tines are exposed, producing a characteristic appearance. 4
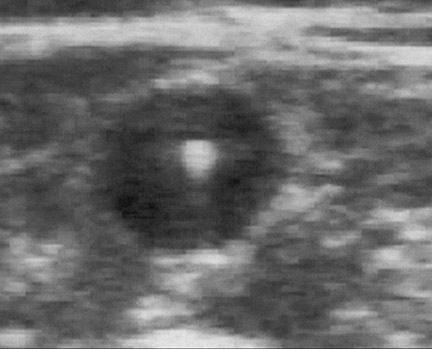
The next step in EVTA is the delivery of tumescent anesthesia (TA), a dilute, large-volume local anesthetic, into the perivenous space of the vein to be treated. Tumescent anesthesia is used not only to make the procedure painless but also to insulate the vein from the surrounding nerves, arteries, and skin as well as to efface the lumen of the vein to maximize circumferential energy transfer to the vein wall. Duplex ultrasound guidance of the needle used to inject the TA fiuid in the perivenous saphenous sheath is essential to maximize the effectiveness and efficiency of its delivery. Successful TA results in a perivenous hypoechoic halo that obliterates the vein lumen from the puncture site to the highest end of the treated segment (Figure 4).
Thermal ablation begins only after DUS is used to confirm once again that the tip of the EVTA tool is at the desired starting point. When the laser fiber is activated, steam bubbles can be seen at the tip of the bare fiber, adding confidence to the position of the tip as seen with US. After this, observation with DUS usually has little utility. Some operators perform DUS immediately after EVTA to confirm success, but the TA and vein spasm usually obliterate the lumen of the treated vessel even without thermal energy delivery. Echo-genic bubbles can be seen in the residual lumen and can sometimes even be seen in the surrounding tissue, but these are of no clinical importance.
Periodic DUS should always be performed to evaluate the treated vein segments after EVTA. 5,7 This is generally done in the first few weeks after therapy, again a few months later, and then at yearly intervals. Duplex ultrasound should also be performed to evaluate for the cause of any recurrent varicose veins. In the first several weeks after therapy, the treated veins will either be smaller or the same size as they were prior to treatment, with a thick wall and nearly obliterated lumen (Figure 5). There should be no fiow in the entire treated vein segment. This should be distinguished from the appearance of a thrombosed vein that in the first few weeks would appear as a central hypoechoic to moderately hyper-echoic filling defect in a vein, which is at least as enlarged as the pretreatment diameter. Partially thrombosed veins may have some eccentric fiow. After several months to a year, successfully treated vein segments will usually be difficult to identify or will be significantly smaller than the vein prior to treatment and will have no fiow. 5,7 Most treatment failures will be evident in the first few weeks as either thrombosed vein segments that subsequently recanalize or as veins that are unchanged compared with the preliminary examination. Most treatment failures are apparent under DUS as a central segment of vein, beginning near the SFJ, or the saphenopopliteal junction in the case of SSV ablation, extending downward to the takeoff of an incompetent tributary. Below this level, the vein is often successfully treated.
Conclusion
Duplex ultrasound is an integral part of the modern evaluation and management of patients with SVI. Precise anatomical and fiow mapping is required prior to planning treatment in all patients with varicose veins. Treatment with EVTA, and occasionally sclerotherapy, also requires precise monitoring with DUS. All patients treated with ETVA should be followed for several months after the procedure to confirm its success. It is incumbent upon all physicians involved in caring for patients with SVI to become familiar with the use of DUS to optimally care for their patients.
Related Articles
Citation
Duplex ultrasound for superficial venous insufficiency: Use for diagnosis and guiding therapy. Appl Radiol.
June 8, 2005