Differentiating Focal Liver Lesions: ICC and HCC
Images
Case Summary
Case 1: A middle-aged adult presented with a history of generalized pruritus followed by development of jaundice one to two weeks after pruritus onset. Laboratory evaluation revealed elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin.
Case 2: An elderly adult with a history of cirrhosis and mildly elevated liver enzymes presented for evaluation of indeterminate liver lesion identified on routine screening examination.
Imaging Findings
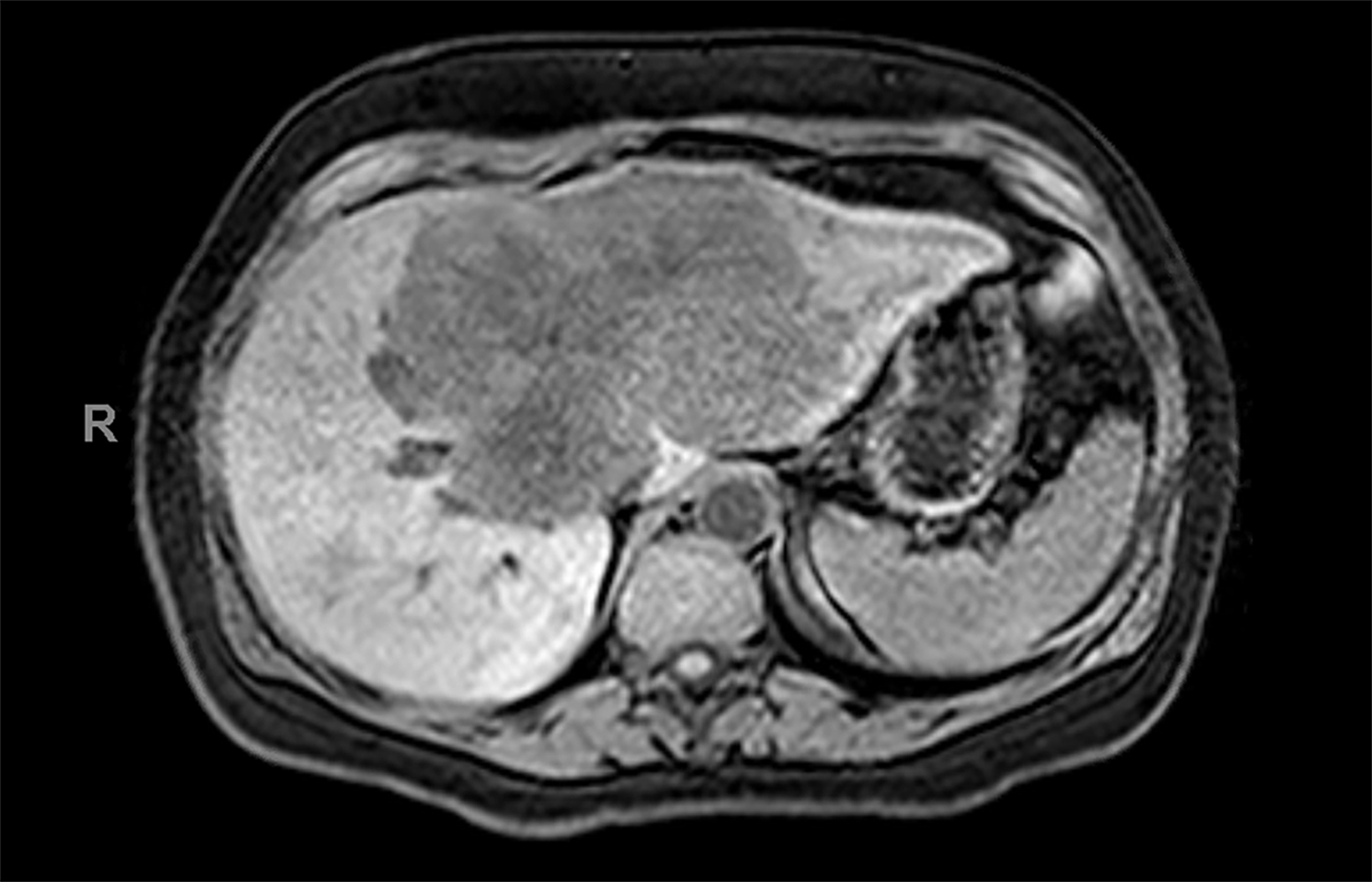
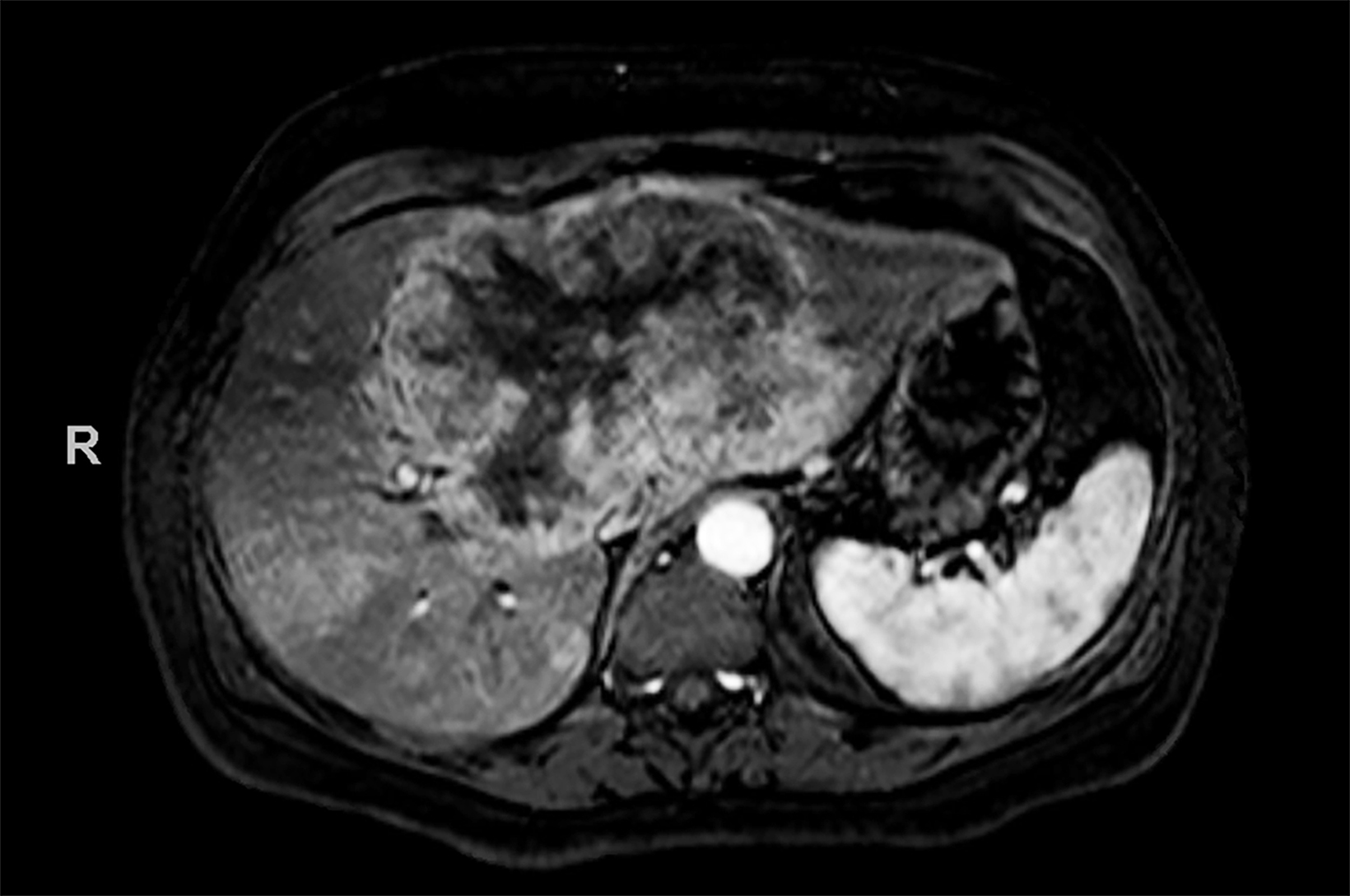
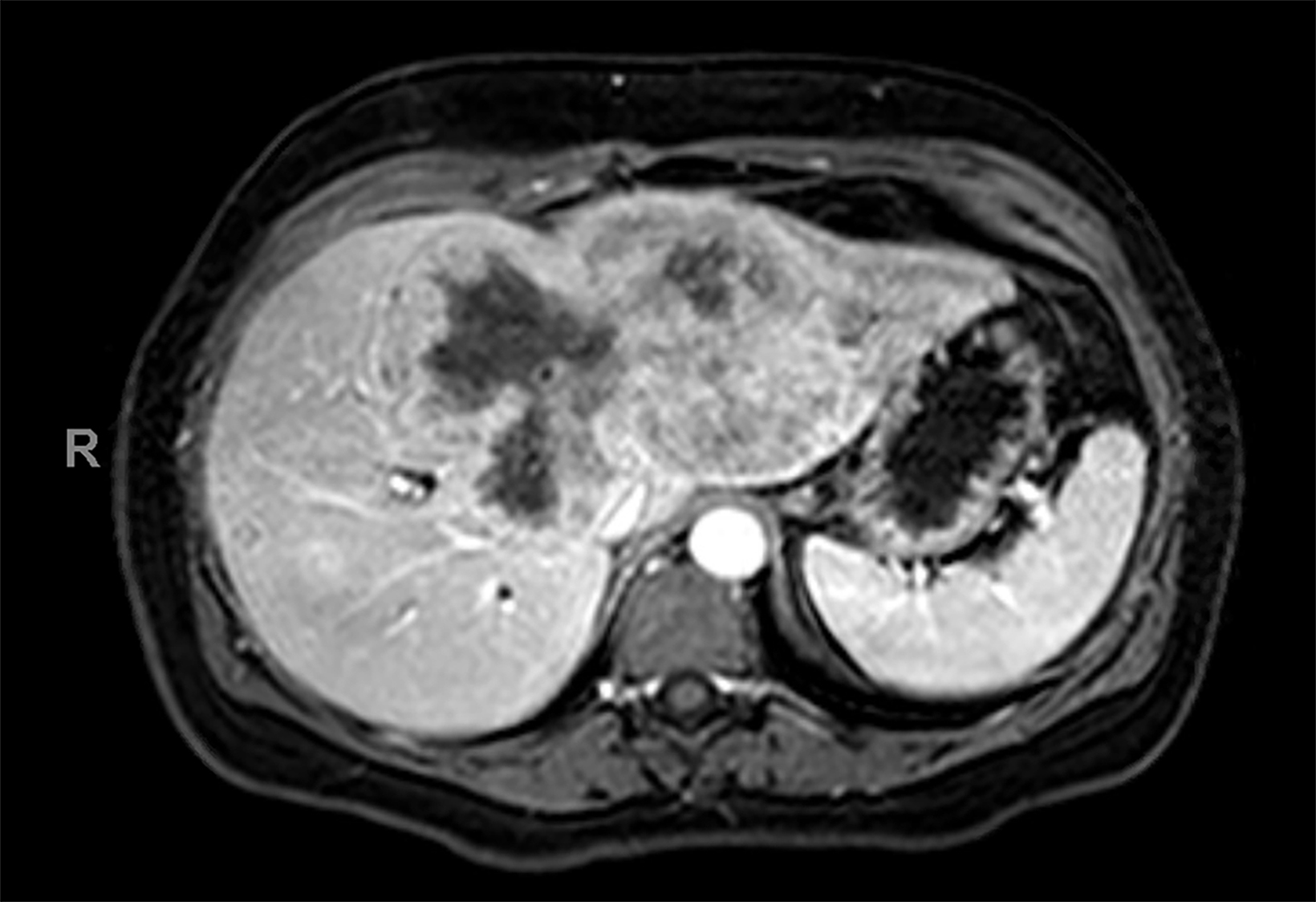
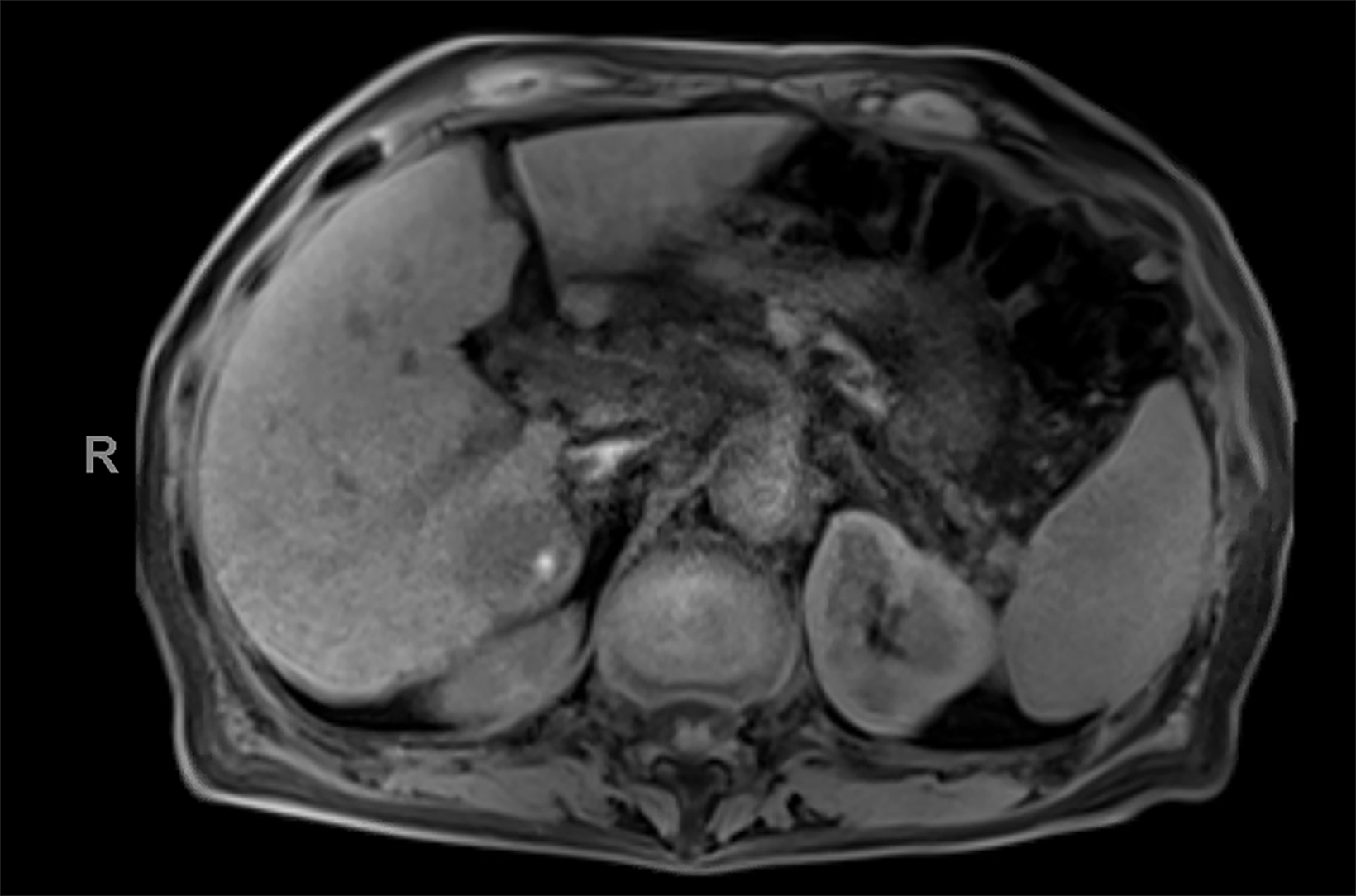
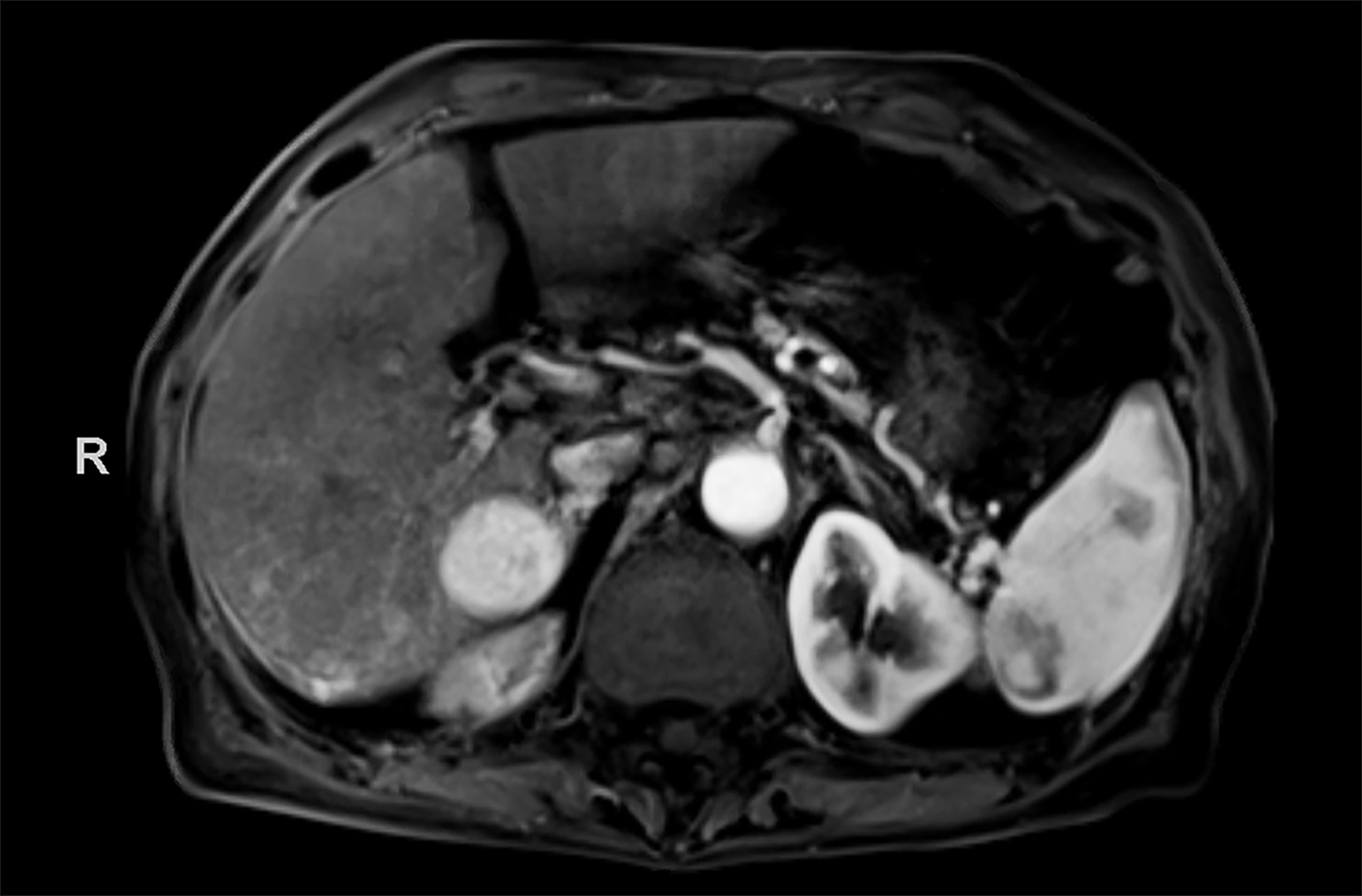
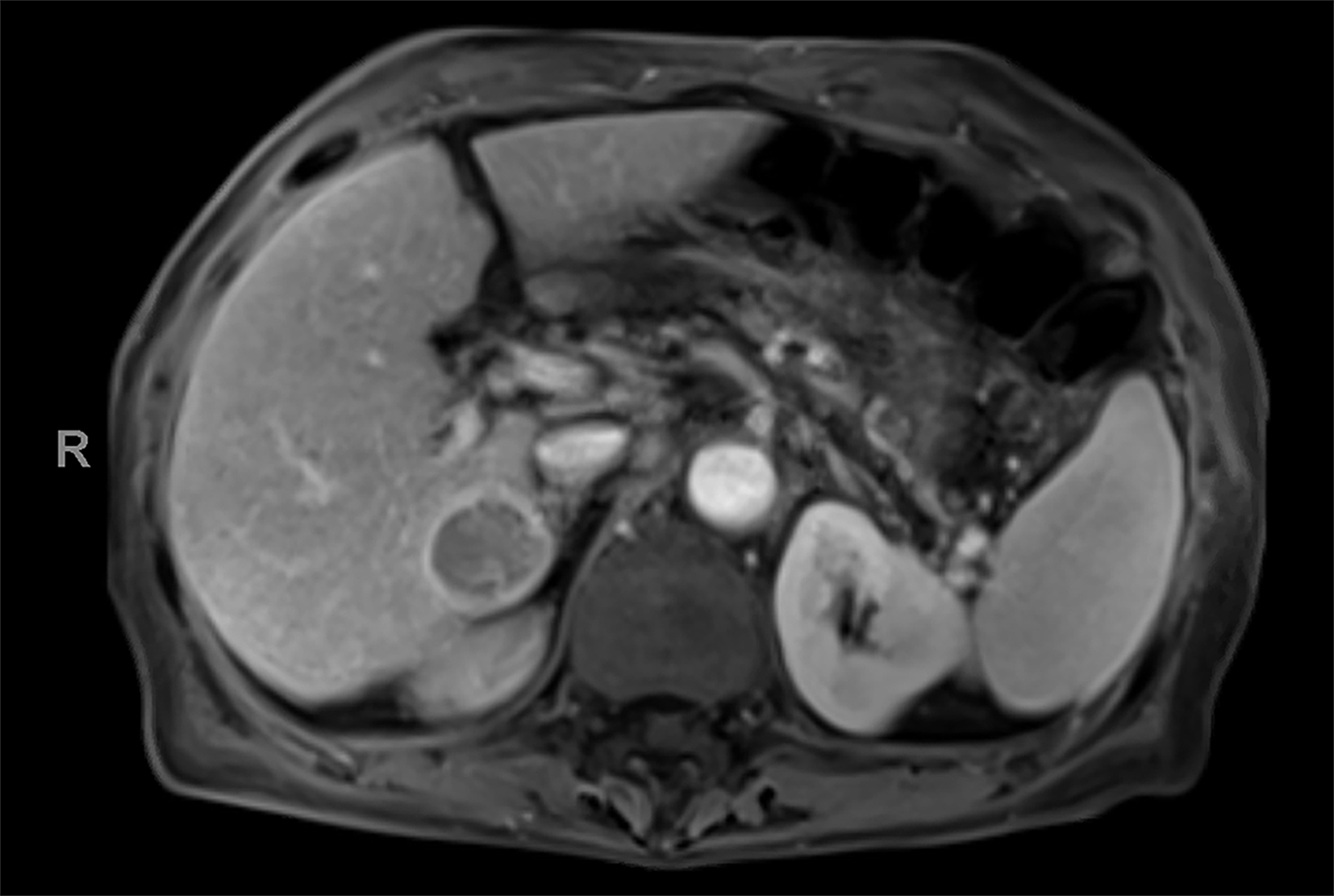
Contrast-enhanced magnetic resonance imaging (CEMRI) of the liver was performed in both cases. In case 1, imaging with an intracellular contrast agent revealed a large mass centered in the left hepatic lobe demonstrating arterial peripheral hyperenhancement with centripetal fill-in on transitional and hepatobiliary phase imaging. In case 2, imaging with an extracellular contrast agent demonstrated a lesion in the inferior right hepatic lobe with non-rim arterial hyperenhancement with central washout and rim enhancement on the portal venous phase.
Diagnosis
Case 1: Intrahepatic cholangiocarcinoma (ICC)
Case 2: Hepatocellular carcinoma (HCC)
Discussion
While ultrasound remains the primary screening modality for hepatic malignancy, CEMRI of the liver is a valuable and complementary tool in evaluating indeterminate liver lesions. Differentiating hepatic lesions in cirrhosis can be challenging, both in part because of altered physiology within the cirrhotic liver, as well as a lack of unique, diagnostic features of various lesions—specifically intrahepatic cholangiocarcinoma (ICC) versus hepatocellular carcinoma (HCC), or combined tumor HCC-CC. There is also significant overlap in patient populations, as cirrhosis is associated with an increased risk of HCC. However, many factors that predispose a patient to cirrhosis are also associated with increased risk of ICC, including viral hepatitis and heavy alcohol use.
MRI contrast agents in hepatic imaging can be broadly divided into either extracellular or intracellular. Extracellular agents allow not only assessment of vascular flow kinetics but also vascular permeability. Iodinated contrast in computed tomography (CT) acts similarly physiologically and provides similar diagnostic information in liver imaging; however, its use may be limited in cases of renal dysfunction.
Intracellular contrast agents in hepatic imaging. meanwhile, distribute into the vascular and extravascular spaces during the arterial and delayed dynamic phases, and then progressively distribute into the hepatocytes and bile ducts during the hepatobiliary phase.
Intracellular contrast agents are often helpful in characterizing liver lesions based on the presence or absence of intralesional hepatocytes, as approximately 50% of the contrast is taken up by functioning hepatocytes and excreted into the biliary system.1 However, as functioning hepatocytes are required for uptake of intracellular agents, patients with cirrhosis can have reduced uptake, resulting in reduced lesion conspicuity.2 Cholestasis can also impact uptake and excretion of intracellular agents, also decreasing lesion conspicuity.
Marked fibrosis and iron overload conditions may result in heterogeneous parenchymal enhancement in the hepatobiliary phase. This reduces background contrast and lesion detectability, particularly in poorly marginated lesions such as infiltrative HCC. In addition, confluent fibrosis can be misidentified as HCC, since both HCC and confluent fibrosis appear hypointense on the hepatobiliary phase.
In contrast to their extracellular counterparts, intracellular agents do not have a conventional delayed vascular phase. Instead, a transitional phase overlaps with the portal venous and hepatobiliary phases. Intralesional signal intensity on the transitional phase is not well understood and has not been sufficiently characterized, potentially leading to misinterpretation.
As such, lesion washout should only be evaluated on the portal venous phase when an intracellular contrast agent is used. Background liver enhancement on the transitional phase also limits sensitivity to capsular enhancement. Additionally, in the arterial phase, intracellular agents have been found to demonstrate weak arterial hyperenhancement at approved doses and may also result in a relatively high frequency of artifact related to transient dyspnea.3
Despite the limitations of intracellular agents in HCC, studies have demonstrated that adding a hepatobiliary phase can improve per-lesion sensitivity for HCC diagnosis by 6%-15%. Unequivocal HCC diagnosis with an extracellular contrast agent requires arterial hyperenhancement with washout or capsule appearance; however, up to 40% of HCC lesions may not be arterially hyper-enhancing. Only lesions with significant neoangiogenesis will demonstrate arterial hyperenhancement with extracellular contrast agents, potentially limiting early HCC detection.
Additionally, poorly differentiated and infiltrative HCC may also demonstrate weak arterial hyperenhancement. For example, small, in- determinate, arterially enhancing nodules are suspicious for malignancy (HCC vs ICC) if, after excluding a hemangioma, the lesion remains hypointense on the hepatobiliary phase.
An intracellular agent can also help differentiate HCC from hypervascular pseudolesions such as focal perfusion alterations, since pseudole- sions enhance similarly to background liver on hepatobiliary phase while HCC is typically hypointense. Although HCC is typically hypointense on hepatobiliary phase, a small subset of moderately and well-differentiated HCC may be isointense or hyperintense on hepatobiliary phase, leading to potential diagnostic confusion. In addition to the diagnostic utility of a hepatobiliary contrast agent, research suggests that intracellular agents may also provide important prognostic information regarding tumor biology, including tumor grade and microvascular invasion.3
Prior studies have demonstrated no unique imaging findings to either HCC or ICC; however, several significant imaging features are statistically more likely to be seen in HCC versus ICC or combined tumor HCC-CC. These include lesion washout, presence of a capsule, and intralesional fat. Features more commonly seen in ICC and combined tumors include peripheral arterial phase hyperenhancement and progressive central enhancement. LI-RADS, the liver imaging reporting and data system, incorporates several of these features into major and ancillary findings to help differentiate HCC from non-HCC malignancy.4
Distinguishing HCC from ICC is useful not just prognostically, but also in guiding patient management. Although pathologic confirmation with tissue sampling is often necessary, imaging plays an important supplementary role. While a solitary HCC lesion may not exclude liver transplantation in an otherwise eligible patient, a solitary ICC lesion may be considered a contraindication to liver transplantation. Researchers, however, have begun to question this approach, noting retrospective data that suggests early transplantation for a solitary ICC lesion up to 2 cm in size may achieve up to 70% 5-year survival.5 Prospective trials may be needed to confirm these findings before a change in standard clinical practice is likely.
Given the importance of lesion size in guiding treatment strategies such as liver transplantation, early detection is critical. MRI or CT surveillance may be indicated in patients for whom ultrasound is limited by body habitus, steatosis, or severe parenchymal heterogeneity related to advanced cirrhosis. MRI surveillance of the liver has shown promise in the PRIUS trial, a prospective cohort study in which MRI provided significantly higher early HCC detection and fewer false positives compared to ultrasound. Given the prohibitive cost and scanning time with routine protocols, some institutions have evaluated the efficacy of an abbreviated MRI protocol and demonstrated higher sensitivity and specificity in early HCC detection.6
Conclusion
Contrast-enhanced MRI of the liver is an important diagnostic tool in the evaluation of indeterminate liver lesions. Though not routinely used for surveillance in high-risk patients, MRI or CT surveillance may be indicated where ultrasound is limited. Knowledge of the various gadolinium-based contrast agents and their physiologic properties and inherent limitations is important in selecting an appropriate diagnostic examination. Liver imaging is critical in guiding development of a treatment strategy for hepatic malignancy. Although pathologic confirmation with tissue sampling is usually necessary, MRI of the liver provides an opportunity for early detection of small lesions and may help differentiate HCC and ICC. Additionally, research has shown the ability to obtain potential prognostic information based on characterization of tumor biology.
References
- Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635-654.
- Kim SY, Wu EH, Park SH, et al. Comparison of hepatocellular carcinoma conspicuity on hepatobiliary phase images with gadoxetate disodium vs. delayed phase images with extracellular cellular contrast agent. Abdom Radiol (NY). 2016;41(8):1522-1531.
- Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30-50.
- Tang A, Bashir MR, Corwin MT, et al. Evidence supporting LI-RADS major Features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: A systematic review. Radiology. 2018;286(1):29-48.
- Mazzaferro V, Battiston C, Sposito C. Pro (with caution): Extended oncologic indications in liver transplantation. Liver Transpl. 2018;24(1):98-103.
- Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: Current best practice and future direction. Gastroenterology. 2019;157(1):54-64.
References
Citation
DS S, KK P.Differentiating Focal Liver Lesions: ICC and HCC. Appl Radiol. 2022; (2):36-38.
March 4, 2022