CT reshapes coronary and pulmonary angiography
Images


Dr. Kazerooni is a Professor of Radiology and Director of Thoracic Radiology at the University of Michigan Medical Center, Ann Arbor, MI.
Cardiovascular disease is the leading cause of death in the United States, responsible for nearly 40% of deaths each year. 1 The combined direct and indirect costs of cardiovascular disease exceed $393 billion annually. Of the lives lost to cardiovascular disease, coronary heart disease claims 53%, making it the leading cause of cardiovascular death. In fact, in 2002, nearly a half-million Americans died of coronary heart disease.
The gold standard test for the diagnosis of coronary artery disease (CAD) is cardiac catheterization. Though effective, this procedure is invasive, expensive, and associated with morbidity and mortality. According to 1990 registry data from the Society for Cardiovascular Angiography and Interventions, the total rate of complications during diagnostic cardiac catheterization was 1.77%, including death, myocardial infarction, arrhythmia, neurological complications, vascular complications, and adverse reactions to contrast medium. 2
In addition, the accepted rate for normal findings on cardiac catheterization, defined as insignificant or absent luminal narrowing, is 20% to 27%. 2 A noninvasive test that could identify patients with normal coronary arteries would be expected to reduce the number of invasive procedures.
As little as a decade ago, noninvasive alternatives barely existed. Today, they are hailed in the popular press as breakthroughs in the evaluation of patients with cardiovascular disease. In January 2004, for example, The Wall Street Journal placed coronary computed tomography (CT) and magnetic resonance imaging (MRI) at the top of its list of 10 major medical advances. The article, "A Better View of Your Heart," 3 noted that rapid advances in CT and MRI technology are changing the rules of angiography, making the tests available not only to patients with symptoms, but also to those with worrisome risk factors.
Accuracy
Given rapid advances in technology, coronary CT angiography (CTA) may soon become a routine part of clinical practice. Previously, with single-, 4-, and 8-row scanners, coronary CTA demonstrated variable degrees of success in detecting critical stenoses, and, as a result, was not considered robust enough for everyday clinical use. 4 That consensus began to shift with the introduction of 16-row multidetector CT (MDCT) technology. The ability to acquire data in submillimeter-thick slices (0.625-mm or 0.75-mm) enabled isotropic imaging in the z-axis and improved spatial resolution. At the same time, faster gantry rotation speeds (0.4 seconds) improved temporal resolution, reducing motion artifacts associated with the beating heart.
Several studies have examined the ability of 16-row coronary CTA to detect CAD. Nieman et al 5 studied 59 patients who were scheduled to undergo elective cardiac catheterization, excluding those with an irregular heart rate. Using a 0.75-mm detector collimation and a 0.42-second gantry rotation, investigators evaluated coronary arteries ≥2 mm in diameter for stenoses of at least 50%. They found that coronary CTA had a sensitivity of 95% for detecting critical stenoses and a negative predictive value of 86% among the evaluable coronary artery segments. The overall accuracy of coronary CTA depended on the vessel. It was very high in the left main and the left anterior descending coronary arteries (100% and 91%, respectively) but was lower in the right and circumflex coronary arteries (86% and 81%, respectively).
A similar study by Ropers et al 6 enrolled 77 patients scheduled to undergo cardiac catheterization, excluding those with cardiac arrhythmias and unstable angina. Using a scan protocol similar to that of the Nieman study, the investigators examined coronary artery branches ≥1.5 mm in diameter for stenoses of at least 50%. Coronary CTA demonstrated a sensitivity of 92% and a negative predictive value of 97% among the evaluable coronary artery segments.
However, these studies do not yet provide enough information to determine the role of coronary CTA in clinical practice. A consistent finding among studies of 16-slice coronary CTA is the large number of nonevaluable coronary artery segments. In the study by Ropers et al, 6 38 of the segments involving 12% of patients were not evaluable because of excessive coronary calcification or motion artifacts.
Imaging protocol
Table 1 outlines the technique we use at the University of Michigan for 16-row coronary CTA. Scanning the entire heart from apex to base (or up through the subclavian arteries, in the case of a bypass graft) takes 15 to 29 seconds, depending on the scanner platform, patient size, detector collimation, and gantry rotation speed.
Since delivery of our 64-row volume CT (VCT) scanner, we use it to perform all coronary CTA. The technique is similar to that described in the 16-row protocol, but data acquisition takes only 5 to 6 seconds, resulting in a more robust dataset and more consistent evaluation of coronary arteries as small as 1 to 2 mm in size.
We use a detector collimation of 0.625 mm, an overlapping narrow pitch (0.2 to 0.35, depending on heart rate), and the fastest gantry rotation possible on our scanner (0.42 seconds). We administer 80 mL of iodixanol (Visipaque, GE Healthcare, Princeton, NJ) 320 mgI/mL, injecting at 4 mL/sec. The amount of contrast material depends on the scan time, the patient's individual physiology, and the scan range. We use a 30- to 50-mL saline chaser bolus to keep the contrast column moving and reduce overall contrast volume. With the VCT, we have routinely reduced our contrast volume to 80 mL.
At the beginning of each examination, we do a non-contrast-enhanced, electrocardiographically gated acquisition to assess the degree of coronary calcification and to map the coronary arteries. The noncontrast acquisition confirms that we have selected the right scan range for the heart and the coronary arteries. This step is particularly important in patients who have undergone coronary artery bypass grafting, as we need to identify the graft's origin before the scan to ensure that it is included in the field-of-view during coronary CTA.
To optimize contrast enhancement, we deliver a 20-mL timing bolus prior to image acquisition, documenting a time-density curve and using the time to peak enhancement, plus 5 seconds, as the scan delay. This delay provides enough time for contrast material to opacify the distal coronary artery branches before image acquisition begins.
Heart rate
Speed and motion control are critical to imaging moving structures, particularly the coronary arteries. Cardiac motion artifacts can be minimized in several ways. One is through faster scanning, made possible by newer-generation CT scanners. In addition, heart-rate control remains a key step in coronary CTA. The faster the heart rate, the more numerous the image artifacts and the less accurate the detection of stenoses. The heart rate can be manipulated through not only the use of beta-blockers but also the selection of contrast material.
An early study by Schroeder et al 7 illustrated the impact of heart rate on vessel visibility. Using a 4-detector-row CT scanner, these investigators found that the patients with the highest number of visible coronary segments had the lowest heart rate, a mean of approximately 60 bpm. They also showed that segment visibility was inversely correlated with heart rate (r = 0.48; P <0.0001).
A study of 100 patients by Gerber et al 8 showed that heart rate influenced the number of coronary segments with motion artifacts. Patients with a heart rate of 51 to 60 bpm had motion artifacts in only 12% of coronary segments, whereas patients with a heart rate of 61 to 70 bpm had motion artifacts in 71% of the segments.
Heart rate can impact not only image quality but also the ability to detect stenoses. In a study of 100 patients who underwent coronary CTA on an 8-row scanner, Giesler et al 9 showed that the sensitivity for the detection of critical stenoses was 62% when the heart rate was <70 bpm, but only 33% at higher heart rates.
Most physicians who perform coronary CTA use a beta blocker to reduce the heart rate to <65 bpm. At the University of Michigan, we use a combination of oral and intravenous metoprolol, depending on the patient's heart rate just before the scan. Occasionally, in patients with very rapid heart rates in whom we need very tight control, we administer intravenous esmolol, a very-short-acting beta blocker.
We also use isosmolar contrast medium for all coronary CTA examinations as a way of minimizing fluctuations in heart rate and blood pressure. Studies by Roriz et al 10 and Tveit et al 11 have shown a greater increase in heart rate with the low-osmolar agent ioxaglate than with the isosmolar agent iodixanol.
An increase in heart rate in response to the administration of contrast medium is more common in patients with severe heart disease, including coronary artery stenosis or prior myocardial infarction. 10 It may occur due to a reduction in blood pressure or to patient stress and discomfort. These and other studies have also shown that iodixanol is associated with fewer patient reports of discomfort and warmth when compared with low-osmolar contrast material. 10,11
Data sets
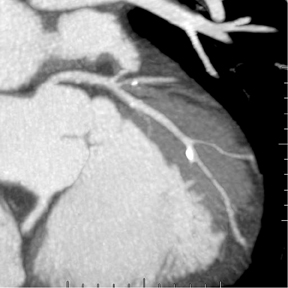
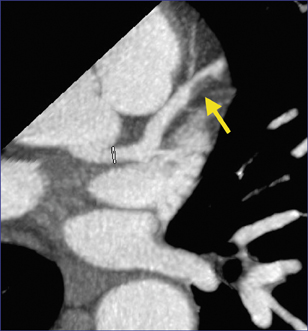
Rather than assigning technologists to do image postprocessing, physicians at our institution interact with the data sets, reconstructing images as part of the interpretation. We generate views of the coronary arteries in multiple planes. Figure 1A shows a curved reformat of the right coronary artery, visible down to a diameter of 2 mm and smaller. Figure 1B shows the left main coronary artery as it branches into the left circumflex and left anterior descending coronary arteries.
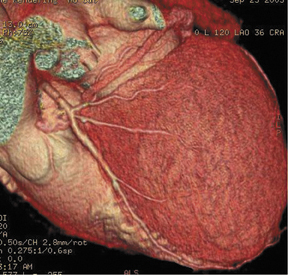
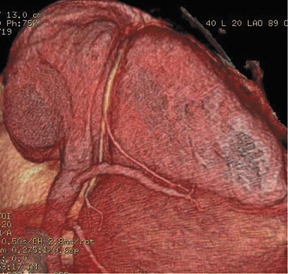
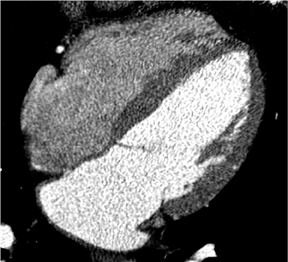
We can also generate surface displays, add color, and create stunning images to send to clinicians and patients. As shown in Figure 2, the result is more than artistry. This surface display depicts very fine anatomic detail in vessels as small as 1 to 1.5 mm in diameter.
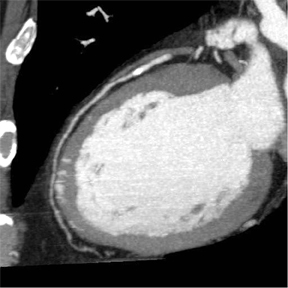
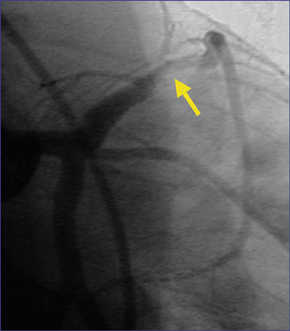
Coronary CTA can evaluate the artery in ways that conventional cardiac catheterization cannot, depicting not merely narrowing of the arterial lumen, but also remodeling and external expansion of the arterial wall. Figure 3 shows a 16-row CTA in a middle-aged hypertensive patient with a family history of coronary artery disease. His coronary calcium score was zero, but contrast-enhanced coronary CTA showed a single, noncalcified, focal eccentric thickening of the coronary artery wall-a soft plaque. Cardiac catheterization confirmed the location of the lesion, and intravascular ultrasound confirmed that it was a noncalcified vulnerable plaque.
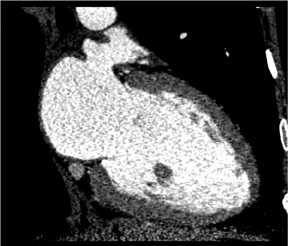
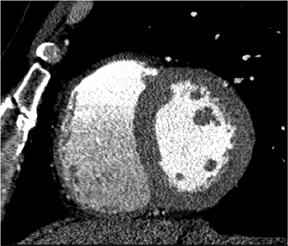
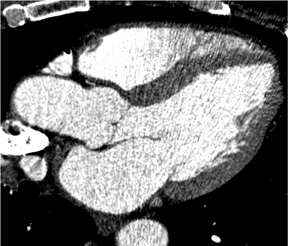
There is more to cardiac CT than evaluation of the coronary arteries. These powerful volumetric data sets contain an enormous amount of information that we can view in very high resolution in any plane. Short-axis, long-axis, 3-chamber, and 4-chamber views are standard in cardiology, whether in echocardiography, nuclear medicine, cardiac MR, or cardiac CT. Figure 4 shows a short-axis view of the right and left ventricles. In the long-axis view of the left atrium and left ventricle, it is possible to see the mitral valve with its leaflets open, as well as the papillary muscles. The 3-chamber view depicts with exquisite detail the mitral valve with its leaflets open and the aortic valve closed during end-diastole.
By adding the element of time to the interpretation of the data set, it is possible to view the heart as it moves through the cardiac cycle. This enables the evaluation of regional and global wall motion abnormalities, as well as quantification of cardiac function through documentation of left ventricular mass, end-systolic and end-diastolic volumes, stroke volume, and ejection fraction.
Role of coronary CT
It is not yet clear what role coronary CTA will play in the evaluation of patients with suspected CAD. Its potential as a screening test must be compared with existing alternatives, such as conventional treadmill and nuclear stress testing. In the future, however, coronary CTA might be used to screen individuals with multiple risk factors and to identify critical lesions.
Coronary CTA may also reshape the diagnosis of CAD and redefine the criteria for vascular intervention. Today, a stenosis of ≥70% is considered to be physiologically significant, warranting bypass grafting or percutaneous coronary intervention. The ability of coronary CTA to detect remodeling and external expansion of the arterial wall raises several new questions. Where does one measure the true coronary artery diameter? How does one define a critical lesion? For example, should a stenosis of 55% in association with soft plaque prompt aggressive treatment?
CT also has the potential to evaluate cardiac function in patients already undergoing coronary CTA. The increased coverage made possible by 64-row scanners may also lend CT the ability to evaluate myocardial perfusion and viability.
Perhaps the most exciting future application of coronary CTA is in plaque characterization and quantification. Closely linked is its potential to guide drug therapy. Patients treated with aggressive lipid-lowering may undergo serial coronary CTA examinations, first to quantify the baseline plaque burden and then to determine whether lipid-lowering medications are effective in reducing that burden.
Pulmonary CTA
Pulmonary embolism (PE) is difficult to diagnose. Physical signs and symptoms are nonspecific, and conventional diagnostic tests are insufficiently sensitive and specific. As many as 70% of ventilation-perfusion (V/Q) scans are nondiagnostic, for example. 12 Even catheter pulmonary angiography, despite being considered the "gold standard," is imperfect. A 1990 study showed that pulmonary angiography had a sensitivity of only 87% for PE. 12
Catheter pulmonary angiography is also underutilized. Studies by Schluger et al 13 and Sostman et al 14 found that, in the majority of cases, physicians chose not to perform pulmonary angiography if the V/Q scan was nondiagnostic.
For these and other reasons, pulmonary CTA is replacing conventional tests. Pulmonary CTA appears to be comparable to invasive angiography for the detection of PE. In a porcine model, Baile et al 15 reported that pulmonary CTA had a sensitivity of 82% and a specificity of 94% for the detection of pulmonary emboli in 1-mm subsegmental vessels. Invasive angiography, by comparison, had a sensitivity and a specificity of 87% and 88%, respectively.
Pulmonary CTA has substantial advantages over V/Q scanning. It enables direct imaging of the thrombus. When combined with indirect CT venography, it identifies other diseases in 25% to 50% of patients. It is highly predictive of patient outcomes. Specifically, a negative pulmonary CTA is associated with a low incidence of PE and deep-vein thrombosis during the subsequent 3 to 12 months. Finally, its interobserver agreement is higher than that of alternative tests, such as the V/Q scan and catheter angiogram. Each of these advantages will be discussed here in more detail.
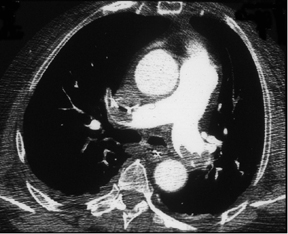
Figure 5 illustrates CTA detection of emboli in the pulmonary arterial circulation. Figure 5A depicts large central pulmonary emboli, and Figure 5B shows nonenhancing right lower lobe atelectasis, which can lead to finding clots that might otherwise have been overlooked.
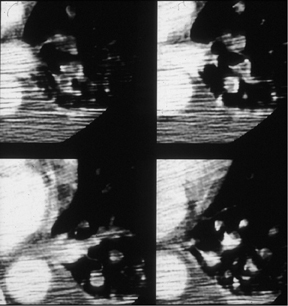
In critically ill ventilator-dependent patients, pulmonary CTA is technically challenging and is often characterized by streaking and image noise. Still, it is possible to identify pulmonary emboli on these images, not just centrally, but even in smaller segmental and subsegmental arteries (Figure 6).
One of the key advantages of CT is that it can detect venous thrombi in the pelvis, legs, and upper extremities, thereby identifying the source of pulmonary emboli and determining whether there are remaining venous thromboemboli that could migrate to the systemic circulation. Detecting clots in the common iliac, infrapopliteal, and other upper- and lower-extremity veins through use of a combined CT angiographic/venographic study can improve diagnostic yield by about 20%, as compared with CTA alone. 16
CT can also detect other disease processes responsible for the clinical presentation in as many as 50% of patients. 17-22 These could include progression of lung cancer, mediastinal fibrosis, or infectious pericarditis, each of which could mimic the signs and symptoms of PE. In addition, CT can reveal other, unrelated findings that are important in the patient evaluation-for example, right heart failure with tricuspid valve incompetence.
The interobserver agreement of pulmonary CTA is better than that of V/Q scanning and probably equal to that of catheter angiography. 23,24 Pulmonary CTA is also highly predictive of patient outcome. An analysis of 8 studies with a combined enrollment of nearly 2000 patients showed that only 1% of patients with a negative pulmonary CTA examination actually developed deep-vein thrombosis or PE during follow-ups of up to 1 year. 25 Thus, a negative CTA study appears to be as predictive of outcome as a negative invasive pulmonary angiogram. 26,27
Imaging protocol
The technique used in performing pulmonary CTA has changed over time, along with a rapid evolution in scanner technology. Acquisition times have gone from 30 seconds to 3 seconds. Coverage has improved, from 12 cm-encompassing only the central part of the pulmonary arterial tree-to more than the 25 to 30 cm needed to cover entire thorax. Collimation has dropped from 5 mm to 0.5 mm. In addition, we can now easily add indirect CT venography to the study.
Advancing technology is prompting an important clinical change as well. With today's 64-slice CT scanners, we are exploring the use of a so-called triple rule-out study for patients with chest pain. In such patients, CTA can determine whether the chest pain is due to coronary artery disease, aortic disease, or pulmonary thromboembolic disease. With 64-slice scanners, it is possible to obtain all of this information in one scan, as long as the imaging technique is tailored appropriately.
Contrast medium
There is an ongoing debate over the best approach to contrast administration in pulmonary CTA. One camp advocates using a fixed scan delay for every patient; another camp advocates tailoring the scan delay to each patient's physiology.
A fixed scan delay is easy and works well in the majority of patients. Using this approach, ≥125 mL of contrast material is injected at 4 mL/sec. Image acquisition begins after a fixed scan delay of 20 to 25 seconds. A fixed scan delay is less successful in patients with poor left ventricular function or another contraindication to a large contrast volume. In such cases, delivery of a small timing bolus should, at least in theory, enable tailoring of contrast administration. The scan delay is the time to peak arterial enhancement plus 4 seconds, to ensure opacification of the entire pulmonary tree.
In my experience and that of other researchers, 28 the use of a timing bolus in pulmonary CTA is time-consuming and less successful than expected. Enhancement of the pulmonary arterial circulation is more variable than that of the systemic arterial circulation, in part as a result of variations in venous in-flow during respiration.
Hartmann et al 28 compared individualized with fixed scan delays and found that image quality was not significantly different. In each case, just over 60% of images were of good quality, 32% to 34% were of moderate quality, and the rest were of poor quality. The mean contrast transit time was 10.5 seconds (range 4 to 26 seconds). Contrast transit time did not correlate significantly with heart rate, blood pressure, patient size, or cardiac function, further confirming that pulmonary arterial enhancement is highly variable.
In selecting a contrast agent, it is important to remember that pulmonary CTA is a first-pass study. It has been suggested that the higher viscosity of isosmolar contrast medium may result in more sluggish flow and improved enhancement of the pulmonary arteries. However, as pulmonary CTA does not involve subsequent acquisitions, higher retained enhancement offers no advantage.
Two recent studies argue against the superiority of isosmolar contrast medium in pulmonary CTA. Goodman et al 29 compared the vascular attenuation achieved when isosmolar and low-osmolar contrast agents were used in CT venography after pulmonary CTA. They found that iodixanol was associated with a modest 7 HU improvement in venous attenuation, but they also found a 42 HU reduction in arterial attenuation ( P <0.05 in both cases).
A separate study by Bedard et al 30 also showed no significant improvement in pulmonary arterial enhancement with isosmolar contrast medium, when compared with low-osmolar contrast medium. Mean pulmonary arterial enhancement was 257 HU and 252 HU, respectively.
Given the small study size and the complexity of pulmonary arterial enhancement dynamics, there may have been too few patients and an insufficient hemodynamic range to demonstrate significant results. Nonetheless, the role of isosmolar contrast medium in pulmonary CTA remains uncertain.
One exception would be in triple rule-out studies using a 64-slice CT scanner. This type of study requires optimal enhancement of the small pulmonary arteries, the coronary arteries, and the aorta all at once. In such a case, isosmolar contrast medium is indicated, as it is ideal for opacification of the coronary arterial tree.
Conclusion
Rapid, recent advances in MDCT technology have created tremendous opportunities for CT imaging in cardiovascular disease. The role of CT in pulmonary embolism and aortic disease has long been recognized. While the exact role of cardiac CT in the evaluation of coronary artery disease has not been fully defined, it is quickly being accepted as a worthwhile test for patients with suspected coronary artery diseases.
Discussion
ELLIOT K. FISHMAN, MD: Cardiac CT is as exciting as it gets. At a course a couple of weeks ago, I asked how many of the 300 people there were doing cardiac CT and there were probably 10 who said they were. I asked how many planned on doing it, and it was about 300. How do you get started in cardiac CT?
ELLA A. KAZEROONI, MD: Well, I think a lot of people want to do coronary CT, and they're afraid to get started. As you said, 10 people are doing it, but 300 want to, that's the reality that I've been seeing, too. There are some review articles in the literature you can read, but I think what you really need to do is to talk to someone who's done it. Pick up the phone and talk to somebody, and I think there are experts around the country who would be happy to help.
Find out what the pitfalls are and get over your fear because, yes, it's a complex exam. Yes, you have to use beta-blockers, which I think is a little bit of people's fear, as they have to set up a nursing protocol to monitor the patients. But it's really not rocket science. There are standard protocols that they can use, there are standard viewing tools for evaluating them, there is a standard nomenclature for evaluating the coronary artery segments, and there are standard ways of giving the beta-blockers. So, I think there is a lot of unnecessary anxiety.
CT radiologists aren't very used to giving medications before a study. So the idea of, "Oh, I'm going to give a beta-blocker and some-body's blood pressure might drop," may be holding people back. But it is just a small dose of an oral beta-blocker 1 hour before a scan or an IV beta-blocker, and having somebody check their heart rate and blood pressure. We haven't had a complication yet. So maybe it's an unnecessary fear--you have to take a protocol, establish it, and start doing it.
JULIA FIELDING, MD: We've found it useful to work with the cardiologists from the beginning, and we actually had a cardiac fellow assigned to us. That did two things: It brought patients down because one of the federal requirements now is that fellows learn about it; and he also took care of the drugs. For a lot of patients, we're trying to make it as joint a service as we can right now to hold it. But is this a reimbursable exam?
KAZEROONI: We've found that we are generally getting reimbursed for patients who have a symptom, for example, chest pain or discomfort, or people who have a known diagnosis of atherosclerotic coronary disease already, if they've had a stent or bypass surgery. What we're not generally getting reimbursed for are the asymptomatic at-risk individuals. While there is going to be a new CPT code for coronary CTA, it will still be some time before it is assigned an RVU value for reimbursement. So, for symptoms or known disease, most people will bill these cases as a chest CTA code.
Now, you could make the case that a coronary CTA could be the equivalent of a treadmill test, but with direct visualization of the coronary arteries. I have heard people say they have gone to their third-party insurance carriers and made the argument that a treadmill test is a screening test, yet you're reimbursed for that. Why aren't you reimbursed for a coronary CTA? It may just be too early, so we don't have enough data to show exactly what the indications are for coronary CTA. But to date, the asymptomatic at-risk people getting screening coronary CTAs have not been reimbursed. So, we have a fixed price for people who are going to get one of these to pay up front.
GEOFFREY D. RUBIN, MD: This is an evolving area, though, because the ACC and the ACR have been getting together about new CPT codes, which are likely going to be what's called Category 3 codes, and will not have RVU values associated with them because the technology at this point probably doesn't meet criteria from the standpoint of proven outcomes to be category 1 codes. So, in fact, there's a real likelihood that in the short term it will get harder to get reimbursed for the test than easier. It will be up to third-party payers how they want to reimburse this code, but there will be no value associated with it from CMS. Right now you can use a thoracic CTA code in many cases with appropriate ICD-9 codes for chest pain and such, and it makes sense. But once there's a dedicated coronary CTA code, then you're going to really have to use that.
FIELDING: I wanted to know also about your triple threat, because I actually feel that's where radiology can work well because we control the ER patients right now. In fact, the cardiologists don't even want to come downstairs to see the ER patients if they possibly can. One of the big problems has been a high radiation dose to the chest if you had to do more than one pass. How do you anticipate doing the triple rule-out with the minimal radiation dose possible?
KAZEROONI: I think if you're willing to keep your contrast volume on the higher side, with one pass you'd be able to get the coro nary CTA, the aorta, and the pulmonary arteries. As long as you keep the volume of contrast flowing so that when you're scanning, everything is opacified, I think you can do it.
FIELDING: That would probably do it.
KAZEROONI: With regard to your comment about the ED, it really depends on local variation. If you have a busy chest pain center, that's often run by the cardiologists, and they are the direct input in the door. The scanners in the hospital are generally owned by the hospital, not by the radiology department or the cardiology department; so, in fairness, any practicing physician who has privileges can have access to any machine they want if they have the privileges to do so. So, simply that we currently do all the CT imaging for the ED does-n't necessarily mean that we would always be doing the ED imaging.
RUBIN: I'd like to just comment on your point about working with cardiologists and, in particular, to have the cardiac fellow give the drugs. I think that there are going to be lots of models that evolve, and people are in a wide variety of practice circumstances where that may or may not be possible. A point Ella made that's very important is that, by and large, giving beta blockers and nitro, which is something else that we do, is a low-morbidity event. It's much better if a radiologist or whoever is going to do the procedure gets over the hump of just saying, "I'm going to give the drugs." Then it's not a problem of having to rely on a crutch of having somebody come down to help them give the drugs because it's just simply not that necessary. There are lots of reasons to work with a cardiologist in this endeavor, but I don't think giving beta-blockers is really one of them.
FIELDING: However, when the patients come down to us, they are often sick, and sick enough that I'm not all that thrilled about having them not monitored by a physician while we're putting them on a scanner. Are you willing to give the beta-blockers and not be present? That would be an issue to me, I think, depending on the sickness level of the patient.
KAZEROONI: Well, for our patients that come down from any of our intensive care units, if they're sick enough to be in the CCU, they come down with what we call our "SWAT team," which involves a nurse, respiratory tech, and, sometimes, a resident as well. But there's always a nurse who comes down with them on the SWAT team.
FIELDING: Then you would write your order for your meds and give it.
KAZEROONI: We write the orders for the meds and our nurses give them. For the outpatients, we have a number of nurses who work in the radiology department, and the number of radiology nurses seems to be increasing every year. The nurses evaluate the patients. They have a standard checklist where they ask patients about contraindications for beta-blockers; they check their heart rate and blood pressure. They give the medication and then check serially over the next 30 minutes. As soon as their heart rates get down to the 60-70-ish window, then we take the patients back to the scanner. We start with a 50 mg dose of oral metoprolol, and then if the oral hasn't worked, we give an IV dose. But we had a patient recently with a heart rate of 120, who was already on oral beta-blockers. Now, for that patient we had to go to the next step, which as I mentioned was an esmolol drip. That is, I know, a fear that many radiologists would have a harder time overcoming. But we went to the ED and got the esmolol protocol. It turned out that several of the radiology nurses are former ICU and/or ED nurses, so they were very comfortable with running the drip, and we had no problem giving it. Giving an oral and/or IV beta-blocker is low risk.
FIELDING: I don't have any problem with oral. I give beta-blockers all the time for renal biopsies, actually, for hypertension and stuff like that. So I think that can certainly be done.
RUBIN: Our evolving thoughts on beta-blockers are kind of interesting. There's a real desire to want to just make it an oral agent and give it to people and have that be satisfactory. But more and more I'm coming to the opinion that oral beta-blockers are probably not worth the time it takes to give them, particularly for outpatients. Because what we do is we bring the patients in an hour ahead, give them the beta-blocker, and then they sit there. Usually, their heart rate does lower. However, the one thing that you can't anticipate from a resting heart rate measurement is the lability of their heart rate once you give the contrast, and it will be labile. So in any setting, but particularly for outpatients, going primarily to IV and skipping the oral could save you an hour of having the patient there and might give more reliable beta blockade that in the end will reverse quicker than giving the oral agent on top of what might be IV. I think this is going to be an evolving trend, but I'm coming to the feeling that it's just easier to bring people in, put them on the table, and give them IV beta-blockers and skip the oral completely.
FISHMAN: I think in the long term probably you're right. We do the same method. We give 50 mg of metoprolol, hook them up, and get their heart rates. Most of the patients convert to the mid-60s by 30 minutes, and I would say that in 1 out of 10, or 1 out of 15, we have to then give the IV. In about 45 minutes, if they haven't dropped to a good mid-60s, we'll just bring them in the room and use IV.
RUBIN: But do you watch their heart rate during the scan?
FISHMAN: Yes, I do. First of all, when we test their heart rate, we have them hold their breath outside, because that, again, could change things significantly. Remember that even doing non-beta-blockers in patients with noncontrast coronary scanning, as soon as the machine makes that noise, the patients get a little apprehensive and their heart rates increase. That's been shown from cardiac cath as well. The pre-injection and postinjection heart rates tend to be within a few heartbeats. So that's worked very well.
I think you're definitely right--it's great that radiologists work with cardiology. But if we want to develop that area of cardiac imaging, we need to take total responsibility for the entire process. The radiologists need to know the contraindications to beta-blockers. So if you're an asthmatic and you're on inhalers, that's a contraindication to beta-blockers. So I think it's something we probably did like the rest of you, you do in-services, you train the nurses, you train the physicians. But unless you're willing to do the good and the bad part of the study, it's not really going to work for us.
I think it's a challenge, but I think 64-slice scanning makes it much easier. On 16-slices, you really had to get all your ducks in order to make the cardiac imaging work. On 64-slices, it is pretty easy. It's pretty easy to get a high-quality data set essentially every time, and the throughput is really terrific. As Geoff said, if you're doing IV and not PO, it should make it a lot easier not to get the timing down perfectly. One thing I wanted to ask in terms of the medication, is do you use nitroglycerine on your patients?
KAZEROONI: We may give a sublingual oral nitro before the study. A year ago we weren't doing that. It's something we've been doing for about the last year.
RUBIN: We do the same, and I was going to mention that the nitrates are another factor that will raise the heart rate because, in order for the nitrates to be effective, you need to scan about 5 to 7 minutes after giving it. Actually, our colleagues in cardiology who did a study looking at coronary vasodilation every minute after giving sublingual nitroglycerine, found that 5 to 7 minutes is really the peak time. So you can time the scan with that intent.
FISHMAN: Do you give the pill or do you use the spray?
RUBIN: We have to give the pill because of pharmacy regulations. We are not allowed to reuse the spray on multiple patients. Obviously, that would be much better, and my preferred delivery. But we would have to use a separate bottle for each patient, so we use sublingual pills instead.
FISHMAN: You give the sublingual nitroglycerine and then you give them the IV beta-blocker?
RUBIN: No. If we need IV beta-blockers, we give those until we have the target heart rate we need. Then we do an unenhanced calci-um-scoring scan. Once that's completed, we give them the nitrates and set up the contrast injection in time for our 5- to 7-minute delay after the nitrates are on board and do the CTA.
FISHMAN: So you'll just wait 5 to 7 minutes for it to take effect. What is the risk? I know cardiologists have done that forever when doing coronary caths. Do you see any issues in terms of changing the degree of stenosis using nitrates?
RUBIN: If anything, I think it's helpful because the idea is in an area of thick stenosis, the coronaries should not be capable of vasodilatation. However, in the more normal zones, it should. So it should accentuate the difference between the normal and abnormal, and you definitely can see the difference in the size of the coronaries.
KAZEROONI: The cardiologists have been doing this routinely for cardiac caths. As we make comparisons of CT to cardiac cath, as far as where the stenosis is or how severe the stenosis is, if the CT is without nitrates and the cath with nitrates, you're going to be comparing apples and oranges, so you want to make the cardiac CT as close to that cardiac cath as possible.
STANLEY GOLDFARB, MD: Listening to this as an internist, the problem I hear is that you're assuming an understanding of the patient's condition and the safety of these types of maneuvers in a given patient. For example, suppose you have a patient with hypertrophic cardiomyopathy that was unknown to the patient and the referring physician, and now you raise the transaortic gradient and the patient has a cardiac arrest. It can certainly happen in the cardiology suite as well, but the clinicians would be prepared for that sort of complication. So, not only do you need to be able to handle the next routine case, but you also need to be able to handle all the unexpected contingencies.
FISHMAN: We've been thinking about using nitroglycerine and we haven't. I agree with you, the image quality does improve, but we were pretty good without it. I think there is no doubt that it's an evolving field.
The other question I had is for this triple study--there is a lot of interest in that, obviously. You say that you increase the volume. What volume would you do for the triple?
KAZEROONI: Well, if you need to cover everything, you have to think of the aorta, the coronaries, and the pulmonary arteries. So you have to go up to what the highest one was, and, typically, that's for the pulmonary arteries, where we usually use 125 to 135 mL. So we go up to that number for the full study.
FISHMAN: But what about the issue with the right side of the heart and the saline push and everything else?
KAZEROONI: I only partially buy into doing the saline push for the right side of the heart. Clearing the contrast out of the right heart may give me a little more information about the right coronary artery. For gated cardiac exams done for other reasons, such as pericardial disease, masse and mapping for ablations, we see the right coronary artery very well. So, I'm not sure I need to clear the contrast out of the right side of the heart, and I haven't found it to really be an issue.
FISHMAN: Our injection protocols for the coronaries are 80 mL of isosmolar contrast at 4 mL/sec and a 40-mL saline push. The studies all use a test bolus, but they are coming out to about a 28- to 30-second delay. We've always found that the saline push is very good for looking at the right coronary arteries because you really do clear the right side of the heart out. When we didn't do that, we often have a lot of turbulent flow, which creates an artifact, and you can often not get a good visualization of the right coronary. The very proximal part, you can, but depending on the coronary set, you have so much artifact over it. That would be the only concern I have for that triple study.
RUBIN: I agree. It also buys you efficiency with the contrast, and it's well established that when you give a saline push, you will get more enhancement per gram of iodine delivered because you've pushed it out of the central and peripheral veins and gotten it into the pulmonary and systemic arterial circulation. Right now, we certainly prefer to use the saline push, too. On this topic, you had mentioned two approaches to doing the pulmonary CTA, the fixed delay and the test bolus, and had mentioned that the test bolus might be a little less reliable. But I would consider a third approach, which is the one that we actually use, which is direct bolus triggering.
KAZEROONI: Yes, we use that too.
RUBIN: The reason it is separate from a test bolus is because the physiologic impact of a 15- or 20- mL test bolus is totally different from running in your primary bolus. I think there's much better association between what you measure in a pulmonary artery when you're actually measuring your primary bolus than there is when you run that little test bolus.
KAZEROONI: We've tried that method for pulmonary artery CTs as well, and we've found that the time it took to move from knowing where we were measuring enhancement to getting to where the scan started was too great, and that it was affecting our pulmonary arterial enhancement too much. Pulmonary arterial enhancement doesn't appear to be an on/off phenomenon, like the aortic/arterial side of the circulation. Especially when the patient's breathing changes, which rushes nonenhanced blood from the legs and abdomen into the IVC and right atrium, diluting things. The contrast really wasn't still on its up-slope; it was going to go down and come back up again. So we found that really didn't work too much.
FISHMAN: Geoff, where are you triggering it? At what number are you triggering?
RUBIN: We trigger off the right ventricle, first off. So we're pretty close to where we start because we scan our pulmonaries from bottom to top. But also, we take a substantial delay after the contrast arrives, so once the contrast arrives, we wait 10 to 15 seconds before we actually start the scans. We don't have an enhancement threshold; it's visual. When you do these cases, they typically go from 40 to 150 or 200 HU. It's not like a test bolus where you have subtle changes. It jumps up very quickly so the actual number isn't so important. You know when the contrast has arrived. But the point is that not only will poor left ventricular function alter your timing, but so will SVC stenoses because of in-dwelling catheters. There are lots of potential problems that can conspire to hurt you. Even if somebody has had chronic PE and has tricuspid regurgitation and is blowing a lot of contrast in their abdomen, all of these variables can impact it. The Valsalva maneuver that a person performs when they take a deep breath can cause the types of problems for arterial opacification that you're alluding to.
KAZEROONI: Right.
RUBIN: So I think the one thing we can do on the patient side to help avoid that is to train them not to Valsalva when they take their breath-hold for the scan because when they Valsalva, what that ends up doing is pushing a lot of unopacified blood.
KAZEROONI: So what volume do you use for your CTA studies?
RUBIN: We use 120 mL.
FISHMAN: I think the one study of angiography where people fail the most, or feel they fail the most, is pulmonary emboli studies. The comment people always make is, "I do the studies the same exact way. One is perfect, the next one is absolutely terrible," and there's nothing any different. So your recommendation would be to use a visual target as it comes in the right ventricle. How many seconds are you waiting?
RUBIN: Fifteen.
FISHMAN: Fifteen seconds, then bottom up.
RUBIN: Right. You know, whether it's bottom up or top down; I don't have a strong opinion about that anymore. It used to make a difference with single-row CT when it was such a long breath-hold. We do it by convention mostly at this point.
FISHMAN: The big thing for us, also, is that you want to move the machine as little as possible because often you have to move to the top, but that'll add another 5 seconds.
RUBIN: It takes a little longer. I will say that as you get to faster scans, if the patient has a period of poor opacification because of a Valsalva--let's say it's 2 seconds--on a 16-row that 2 seconds represents a lot more anatomic coverage than it does on a 4- or an 8-row scanner; on a 64-row, it's even more. So, with the faster scanners, it's more of an all or none--good study or bad study. On a 4-row, you had a little band that was unopacified. Now, half the chest is potentially unopacified. So that's where the risk comes in.
FISHMAN: But I think that's when people speak about how they overread PA studies because they look and everything's white, white, white, and there's a couple of slices where things aren't so white. It's not like a thrombus there. It's just the relative lack of good enhancement. So that's probably very critical.
In terms of postprocessing, one of the key things I would agree with is your comment that right now the best way of doing a coronary artery study has not yet been determined. Whether it's axials or curved coronals, MIP and volumes, and we use a little bit of everything.
KAZEROONI: Right. We do, too.
FISHMAN: So, do you have a recommendation of how you would tell someone how to interpret a routine coronary study?
KAZEROONI: The first thing I do is to just quickly scroll through the noncontrast axial images to get a sense of the calcium burden because that's going to impact whether I may even be able to interpret the coronary CTA. If you've got a calcium score >1000, I may very well not be able to interpret the coronary CTA in a lot of areas, so it gives me a road map.
FISHMAN: If someone has a very high calcium score, and it's incredible calcification, are you still doing the CTA?
KAZEROONI: Well, since calcium scoring has to be processed after the fact, after the scan has been acquired, we don't say, "No, we won't do the coronary CTA."
People have talked about if a calcium score is over a certain number, if you see a certain amount of calcification, you shouldn't do the coronary CTA. I haven't come to the point where I've said I won't do it if there's a lot of calcification.
But it gives me a sense of what I'm up against when I go to look at the coronary CTA. Then I start with curve reformats, and I like to move the volume around and interact with the data set and try to draw out the small coronary arteries using the curve reformats. I also have a lumen view running so I can stretch out the artery and rotate it and look for whether the stenosis is excentric or circumferential. The other thing I like to have is the cross section, to be able to see the cross section there, so I've got the length of it and I have the cross section together, and I like to have both to combine. All the fancy shaded-surface displays and 3D looks are pretty. They look nice in talks; the cardiologists and their patients like them. But I don't think that's really where the diagnosis is. That's just, "Hey, we've got a beautiful study here today." But I think needing to have both the cross section and the long view of the artery is important.
FISHMAN: When you interpret the study, what do you say? Your final report will be, "Oh, just a normal case." But do you measure stenosis? Do you try to quantify above 50%? Under 50%? How do you report it?
KAZEROONI: We try to report it the way our cardiology colleagues do, to use a similar style as they would in their cardiac cath report.
We measure diameter, percent diameter stenosis. It's not the area of stenosis, but in our facility, most cardiologists report percent diameter stenosis. So that involves either manually or using some software to do it for you, determining what the diameter of the vessel is above the stenosis, below the stenosis, and in the stenosis. By knowing above and below, you estimate what it should be at the stenosis, and then from there you determine the percent diameter stenosis. We report anything >30% stenosis; anything <30% stenosis, we say that there's plaque there, but we don't quantify it.
RUBIN: One of the unique aspects of reading these cases is dealing with the temporal domain. It's absolutely critical to move through the different phases to find the right view for the different coronaries. So our PACS, which is fairly rigid in that you can either show all the images sequentially or by table position, doesn't work for that. You might think that looking by table position would be a good way because you look at 10 phases at one position, and then you go to the next, but it's not a very logical way to navigate.
So we use a workstation exclusively for these cases that allows us to bring up a volumetric view. It might be volume rendering or multiplanar reformation. Then from any view we can click through the 10 phases, and I think that gives the optimum access to what can be up to 4000 images. I probably have a pretty antiquated approach. I feel most comfortable looking at cross sections, and I start out with axials and find the most motion-free views for each vessel and work my way down. I turn the planes so that I'm always perpendicular to the vessel, and it's really a manual turning. I occasionally go parallel to the vessel. Diagnosing intrinsic stenosis and myocardial bridging, which comes up a fair bit in these patients, is really best seen on those kinds of views. I love to create the volume rendering and they're like a reward at the end of the case. But rarely are they particularly useful diagnostically, except perhaps for aberrant coronaries. That's when you really like to have the volume renderings.
FISHMAN: What about MIP?
RUBIN: I find MIP almost worthless, unfortunately. The problem is that you start dilating out a little bit, and, even on a thin-slab MIP, any calcium wipes you out, and the myocardium starts to get in the way. I just really believe that cross sections are the way to go. If I had a convenient way to do curves on my workstation in real time, I would probably use those. If I could create one curve and then have it interpolate across all 10 phases and click through those 10 phases, that would be a pretty swift way to go.
KAZEROONI: I think what you're speaking to is that the software to evaluate the cardiac CTs is much more primitive than the high-tech scanners that generate them. We generate this very powerful data set, but the software tools have really lagged behind a way to efficiently evaluate this data.
RUBIN: You have to deal with what you have. I'd also like to note that it's very unusual for me to measure a stenosis just because I don't think I can believe the measurements. If you look at the number of pixels spread across the coronary, than the best I can hope to do is say whether it's <50% or >50%. If I think it's high grade, ≥90%, I will say that. If it's occluded, I will say that. But I try not to measure.
FISHMAN: What you said is very true. We're doing cardiac imaging, which is the most demanding vascular imaging, but we're doing that with tools that were developed for looking at the abdominal aorta.
I think one of the things we will all see this year will be dedicated cardiac packages that are really optimized cardiac visualizations, whether it's spider views or whatever else. A number of vendors are working on these. That will be a major factor in cardiac imaging.
I think it's a very exciting area. Some of the things you mentioned, such as the functional valve imaging, are just going to continue to grow in that area.
RUBIN: I would like to add one point. I've found that the greatest impact on getting our referrals was giving internal medicine grand rounds. Immediately after the grand rounds, we saw our referrals bump up substantially, on the order of 4 to 5 cases per week. You can reach a lot of key referrers through that one presentation.
KAZEROONI: You said you did calcium scoring first, which is similar to us, and we have the coronary CTA. Do you examine cardiac function on every case? Do you provide ejection fraction?
RUBIN: We want to and we will, once the software is reliable for it. We have had some problems with the nuts and bolts of the DICOM data coming from the CT scanner meshing with the software products that we have to do it. But then we will do that.
FISHMAN: We have one of our faculty doing cardiac function on nearly everybody, and we're doing valve analysis on every patient. We're at the point now where we can compete in this arena, so you have to do more than they ask for. It reminds me of that TV commercial where they show a kid mowing lawns and he always gave customers more than their money's worth--he even manicured the lawns and bushes.
That's what we need to do. Even when they just ask us to report on the coronaries, we're giving them the AVIs and looking at the aortic valve. That's generated a lot of business, for example, from the cardiothoracic surgeons, looking at ascending aortas, looking at valve motion, looking at the coronaries at the same time, and trying to bypass other exams. It's critical for us, if we want to develop that area, to do more than they ask for, show what you can do do--as we've done everywhere in radiology. It is definitely time consuming. The biggest challenge to radiology is for people to be willing to put the time in to do it, from being able to give medication safely, to learning all the postprocessing steps and providing the information.
CTA Panel