CT evaluation of gastrointestinal tumors: Imaging techniques and key diagnostic findings
Images


Dr. Kottler is a Postgraduate Year 5 Resident, Dr. Aguirre is a Research Assistant, and Dr. Sirlin is an Assistant Clinical Professor, Department of Radiology, University of California San Diego, San Diego, CA.
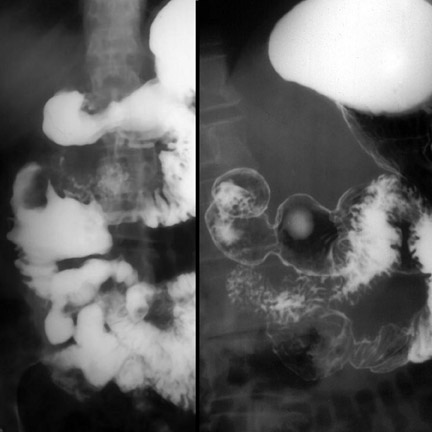
Computed tomography (CT) has become the mainstay for noninvasive diagnosis and staging of many gastrointestinal (GI) tumors. With proper technique, CT permits evaluation of the bowel wall and extramural tissues (Figure 1), a distinct advantage over conventional fiuoroscopic and radiographic examinations that primarily portray the mucosal surface (Figure 2). 1 Depiction of the bowel wall and extramural tissues affords accurate assessment of lesion size and is essential for tumor staging. This article will review: 1) optimal imaging techniques and interpretive pitfalls in GI tumor imaging; 2) imaging features that help differentiate benign from malignant pathology; 3) characteristic findings and complications of common GI malignancies; and 4) patterns of tumor dissemination.
Technique
Optimal technique is essential for accurate detection and staging of GI tumors. A fundamental requirement is luminal distention, 2 requiring the administration of enteric contrast agents (typically approximately 450 mL of enteric contrast given 1 to 2 hours before the CT examination). Historically, barium- or iodine-containing liquid preparations were used. These preparations increase the attenuation of the bowel lumen and are called positive contrast agents. One disadvantage of positive contrast agents is that they interfere with mucosal visualization. More recently, several investigators have advocated the use of hypoattenuating or negative contrast agents, such as water, milk, 3,4 or synthetic preparations. These agents permit direct mural evaluation after intravenous (IV) contrast administration.
If luminal distention is inadequate, one of several ancillary maneuvers should be attempted: 1) delayed imaging to permit passage of enteric contrast into the region of interest; 2) administration of additional oral contrast and/or effervescent material to distend the proximal GI tract; 3) rectal administration of gas or enteric contrast material to distend the colorectum; and 4) patient repositioning to shift intraluminal material.
Unless it is contraindicated, IV contrast material should be used, as it helps to depict and characterize the primary tumor, assess normal bowel wall, stage metastatic disease, and detect complications. 2 Typically, a 125- to 150-mL IV solution (approximately 40 g of iodine) is administered at a rate of 3 to 4 mL/sec. For most GI tumors, venous-phase imaging is sufficient, as these tumors and their metastases tend to be hypovascular. However, some GI tumors, particularly those of mesenchymal and neuroendocrine origin, are hypervascular. If such a tumor is suspected, arterial-phase imaging should also be performed.
Specific imaging parameters are institution- and scanner-dependent. The authors obtain 5-mm images on a 4-slice CT multidetector scanner and, in select cases, retrospectively reconstruct overlapping 2.5-mm images to increase detection of small nodes and peritoneal implants. If necessary, multiplanar reformations are performed.
CT colonography is a specialized procedure for polyp screening. 5-7 Discussion of CT colonographic techniques is beyond the scope of this review.
Bowel-wall thickening: Differentiating benign from malignant GI pathology
Normal bowel-wall thickness is ≤ 3 mm. 2 On CT, bowel-wall thickening >3 mm is a frequent finding. The most common cause is pseudothickening related to incomplete distention. Assuming adequate luminal distention, wall thickening connotes important pathology. The differential diagnosis is broad and includes both neoplastic and non-neoplastic processes. Careful analysis of 4 CT features of the thickened bowel wall usually permits reliable differentiation: 1) bowel-wall attenuation and enhancement, 2) degree of wall thickening, 3) length of involvement, and 4) morphology. Other important clues include distribution of bowel involvement, clinical presentation, patient demographics, and ancillary findings (Table 1).
Bowel-wall attenuation and enhancement
The bowel-wall enhancement pattern helps in the differentiation of benign from malignant disease. Bowel-wall stratification (target sign or double halo sign), in which smooth, alternating low- and high-attenuation layers conform to the circumferential strata of the bowel wall, indicates benign pathology. 2,8 One important exception is scirrhous adenocarcinoma, a tumor that infiltrates the submucosa and muscularis but spares the mucosa. 2 By comparison, heterogeneous wall attenuation and enhancement, in which low-attenuation and high-attenuation areas do not conform to the bowel-wall layers, is suggestive of malignancy. 2 In these cases, the hypoattenuating regions probably represent ischemia, necrosis, and/or accumulation of mucinous material. Homogeneous bowel-wall enhancement is indeterminate, and other factors (below) need to be considered. 9,10
Degree of bowel-wall thickness
In general, mild bowel-wall thickening (<1 cm) suggests benign pathology, 2,8 although early malignancies may have a similar appearance. Marked bowel-wall thickening (>2 cm) suggests malignancy, 8,11 although certain benign conditions (especially mycobacterial infection, cytomegalic virus infection, Crohn disease, and severe ischemic colitis) may also cause marked wall thickening. Wall thickening between 1 and 2 cm is abnormal, but not specific for benignity or malignancy. 9
Length of involvement
Pathology that affects >20 cm of bowel suggests a benign process. 8 Wall thickening that involves <5 cm of bowel suggests malignancy. 2,8 Pathology affecting between 5 and 20 cm is nonspecific, as many malignant (eg, non-Hodgkin's lymphoma [NHL]) and benign (eg, granulomatous disease and bowel hemorrhage) processes cause segmental bowel involvement. 9
Bowel-wall morphology
Wall thickening that is homogeneous, symmetric, smooth, and tapered suggests a benign etiology. 8 Wall thickening that is irregular, asymmetric, eccentric, and abrupt suggests malignancy. 8 A special morphologic pattern that merits further discussion is cavitary wall thickening.
Cavitary masses
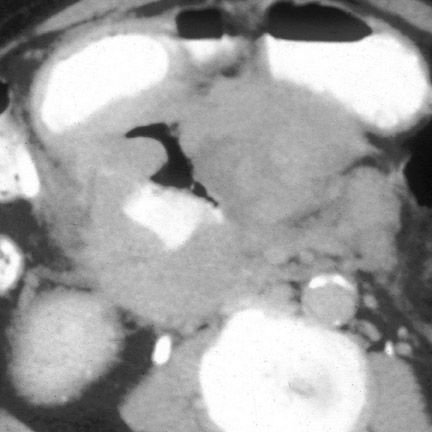
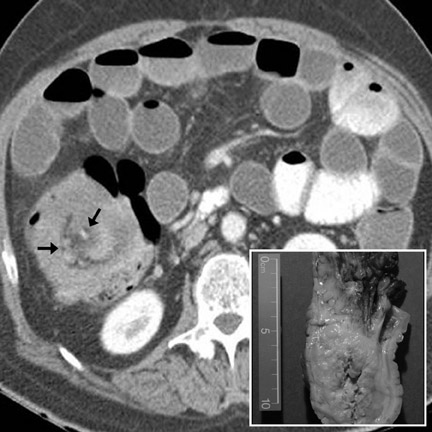
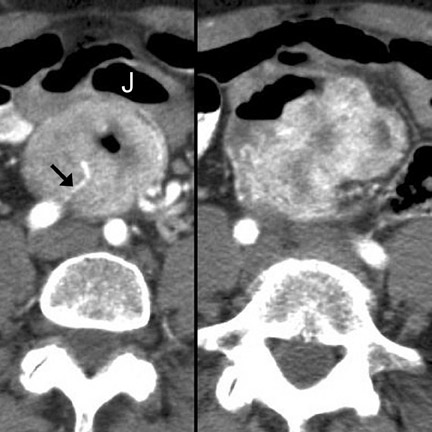
Cavitating masses of the GI tract are an uncommon but important morphologic pattern. The differential diagnosis includes adenocarcinoma, NHL, gastrointestinal stromal tumor (GIST), hematogenous metastases (especially from melanoma), and diverticulitis with abscess formation. These causes are often indistinguishable on CT (Figure 3). Factors favoring malignancy include wall thickness >2 cm, wall enhancement, wall irregularity, bulky or necrotic lymphadenopathy, distant metastasis, and an insidious clinical presentation. 9
Primary neoplasms of the abdominal GI tract
The most common primary neoplasms of the GI tract are adenocarcinoma, lymphoma, and carcinoid and mesenchymal tumors. In recent years, a fifth tumor type, squamous-cell carcinoma of the anus and rectum, has emerged as an important entity in anoreceptive homosexual patients who have tested positive for human immunodeficiency virus (HIV). This topic is being actively investigated and is not further discussed in this review.
Adenocarcinoma
Adenocarcinoma is the most common malignancy of the abdominal GI tract and can arise anywhere from the stomach to the rectum.
Sites of involvement- In descending order, the most frequent sites of adenocarcinoma involvement are: Colon/rectum (Figures 4 through 6), stomach (Figure 7), duodenum, jejunum, ileum, and anus. 2,12,13
Adenocarcinoma is extremely common in the colon and rectum and accounts for well over 90% of colorectal cancers. 14,15 The ascending colon is involved in 25% of cases, the transeverse colon in 10%, the descending colon in 10%, the sigmoid in 30%, and the rectum in 25%. 16 In immunocompetent patients, primary rectal malignancy other than adenocarcinoma is rare; thus, in the absence of HIV or another other immune deficiency, a rectal mass is considered adenocarcinoma until proven otherwise. 16
Gastric adenocarcinoma classically arises in the distal stomach. For reasons that are still unclear, the incidence of proximal disease is increasing. 1,17 This increase is unfortunate, as proximal lesions have a worse prognosis.
At autopsy, adenocarcinoma is the second most common malignancy of the small bowel after carcinoid. 12 It is more common than NHL in the duodenum 13 and jejunum, but less common than NHL in the ileum.
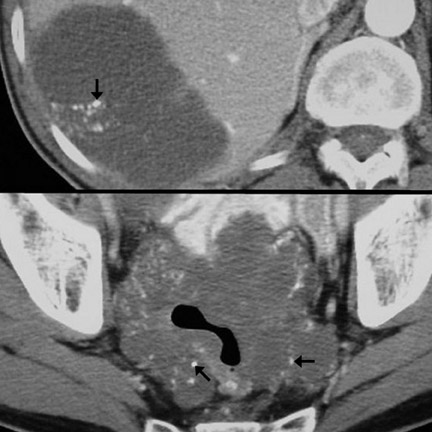
Imaging features- Imaging features characteristic of GI adenocarcinoma include irregular and asymmetric wall thickening, luminal narrowing with tendency for obstruction, and short-segment involvement with an abrupt transition from unaffected bowel wall to mass ("shouldering," Figure 5). 13 Locoregional lymph node (LN) enlargement (Figures 4 and 7) is characteristic. 13,18 Mucinous adenocarcinoma has a tendency to calcify. 18 Such calcifications can be seen in both the primary mass as well as metastatic lesions (Figure 6). Other CT appearances of adenocarcinoma include a lobulated, polypoid mass (Figure 8), superficial nodular mass, and deep ulcerating mass. 12,13,16 Gastric adenocarcinoma may also diffusely infiltrate the submucosa (linitis plastica), causing a rigid or fixed appearance (Figure 7). Differential considerations are provided in Tables 1 and 2 12,13,16
Complications- Complications from adenocarcinoma are frequent and include metastasis (most common), bleeding, bowel obstruction, intussusception (Figure 8), direct invasion into adjacent structures, visceral perforation, fistula formation, and pseudomyxoma peritonei from perforation of mucinous adenocarcinoma. 19
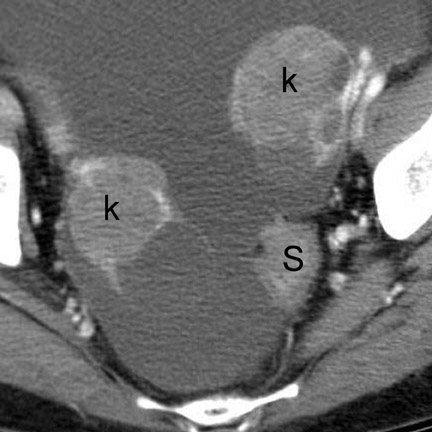
Patterns of spread- Adenocarcinoma of the GI tract can spread to other sites via several mechanisms. Hematogenous metastases usually involve the liver and, less commonly, the lungs or bones. 15 Regardless of location, hematogenous metastases are typically hypovascular and are best seen on portal venous-phase imaging. 20 Lymphatic metastases (Fig-ures 4, 5, and 7) are common, involving locoregional LN first and more distant LN subsequently. 21 Another important mechanism of spread is direct invasion of adjacent structures, including regional fat, omentum, and viscera. Exfoliation of tumor cells into the peritoneum can occur, usually after serosal penetration, and can lead to drop metastases to ovaries (Figure 9), peritoneal and omental implantation, and malignant ascites. 15,21 Carcinomatosis, or diffuse infiltration of peritoneum by adenocarcinoma, can occur in late stages of lymphatic or exfoliative dissemination.
Lymphoma
The GI tract is the most frequent extranodal site of lymphoma. 22 In this location, NHL is much more common than Hodgkin's disease, 13,22-24 and its incidence is rising. 22
Sites of involvement- In immunocompetent patients, the most frequent sites of primary GI involvement are, in descending order, stomach (50%) (Figure 10), ileum (Figure 11), jejunum, duodenum (small bowel 33%), and colon (16%) (Figure 12). 13,22-24 In immunodeficient patients and those of North African, Middle Eastern, and Mediterranean descent, small-bowel involvement is more common than gastric involvement. 22 Anorectal involvement is distinctly unusual in the absence of HIV. 22
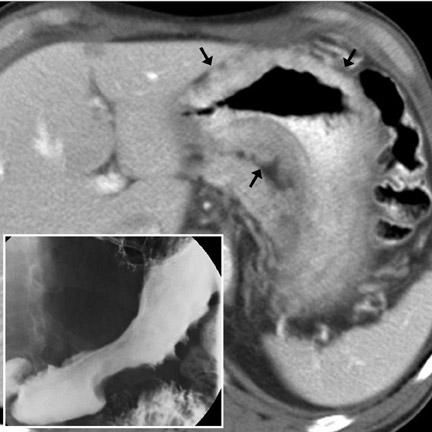
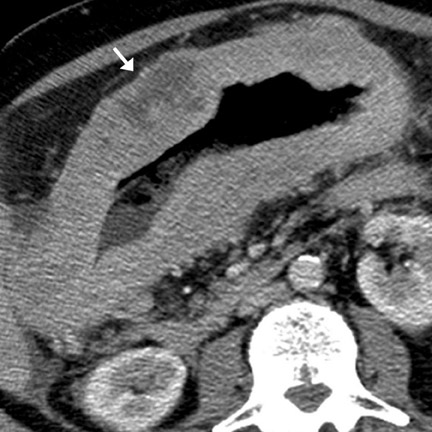
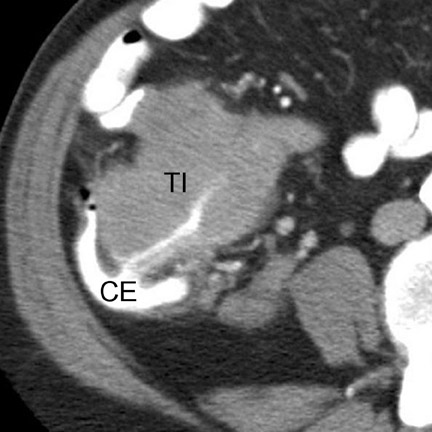
Imaging features- Characteristic CT features of primary GI lymphoma include circumferential, homogeneous and smooth bowel-wall thickening usually without obstruction (Figure 12), mid-to long-segment bowel involvement (Figure 11), bulky LN, which may surround mesenteric vessels ("mesenteric sandwich"), aneurysmal dilatation of involved bowel (Figure 1), and splenomegaly (40%). 2,9,13,22,23 In larger tumors, mural hemorrhage and necrosis may cause heterogeneous bowel-wall attenuation (Figure 10). In the stomach, lymphoma may diffusely infiltrate the submucosa, causing linitis plastica. Differential considerations include adenocarcinoma (Figure 7) (Table 1), GIST (Table 1), metastases, and infiammatory disease (mycobacterial infection, or Crohn disease). 22,23
Patterns of spread- Non-Hodgkin's lymphoma is usually a systemic disease; characteristically, LN, spleen, and other organs are involved at presentation. 22
Complications- Other than diffuse dissemination, complications from lymphoma are less frequent than from adenocarcinoma. Complications include bleeding, intussusception, bowel obstruction, and perforation (which may be silent). 13,23,24
Carcinoid
Carcinoid is a submucosal tumor of neuroendocrine origin.
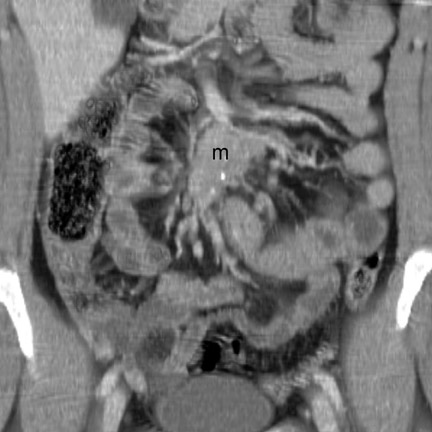
Sites of involvement- GI carcinoid most frequently arises in the appendix (35%) or distal ileum (16%) and hence usually manifests in the right lower quadrant. 2,13 Less common sites include the rectum (13%), colon (3%), proximal small bowel (rare), and stomach (rare). 12,13 Tumors of the stomach, appendix, and rectum frequently have low malignant potential and high 5-year survival rates. 24,25 Tumors of the ileum and colon are often highly malignant with metastatic disease found at the time of presentation. 13,24
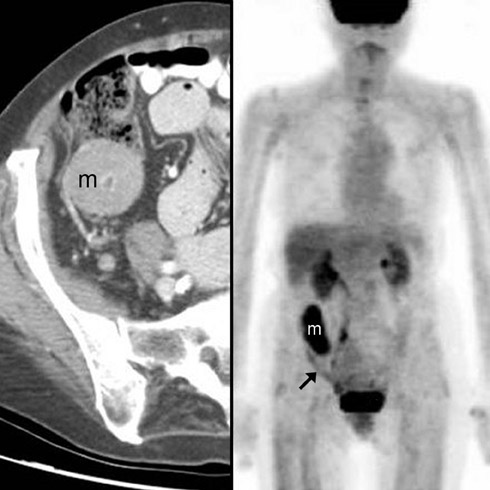
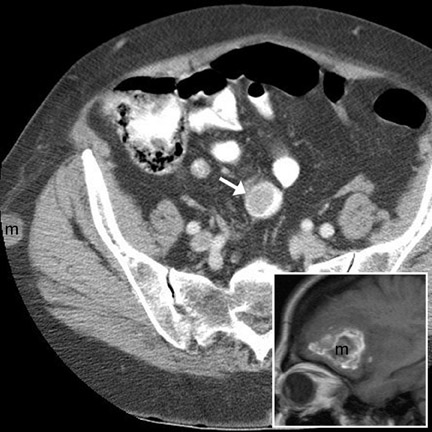
Imaging features- The primary mass is usually undetectable. 12 Occasionally, a submucosal mass, possibly with central ulceration (bull's-eye lesion), may be visible. 17 The classic CT imaging manifestation of carcinoid is a metastatic mass in the regional mesentery (Figure 13). This mass is often hypervascular, partially calcified, and spiculated. 2,12,13 Serotonin secreted by this mass typically incites a desmoplastic reaction, 13,25 leading to lymphatic and venous obstruction and mesenteric and bowel-wall congestion. 13 Dif- ferential considerations include retractile mesenteritis, lymphoma, granulomatous infection, sarcoid, metastasis, GIST, in-fiammatory pseudotumor, and fibromatosis (desmoid). 12,13,26
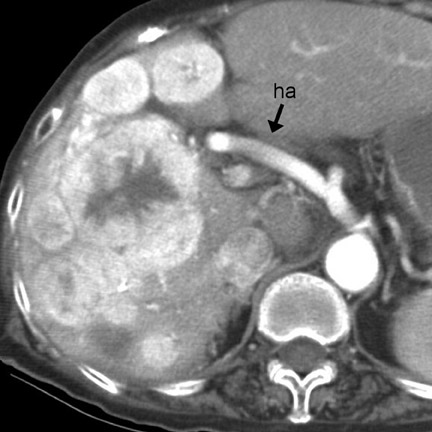
Patterns of spread and complications- Frank metastatic disease is usually present once the primary tumor reaches a diameter >2 cm. 13 In this setting, local metastases to the bowel mesentery (Figure 13) and hematogenous metastases to the liver (Figure 14) can be identified by CT. Classically, the metastatic deposits in the liver are hypervascular (Figure 14) 13 and may lead to carcinoid syndrome (episodic cutaneous fiushing, diarrhea, and bronchospasm). 13 Other complications include intussusception (intraluminal protrusion of primary tumor) and bowel obstruction (fibrotic reaction to the serotonin produced by the mesenteric mass). 13
GIST, leiomyoma, leiomyosarcoma
Tumors of mesenchymal origin in-volving the GI tract include GIST, leiomyoma, and leiomyosarcoma. Gastrointestinal stromal tumor, the most common GI mesenchymal tumor, is composed of several mesenchymal elements. 15 Benignity and malignancy are determined by tumor size (stratified by organ of origin) and histologic factors (eg, mitotic number). 24 In general, larger masses and those involving the colon and rectum are more frequently malignant. 11,12 True leiomyomas and leiomyosarcomas, which are composed purely of smooth muscle elements, are rare. 15
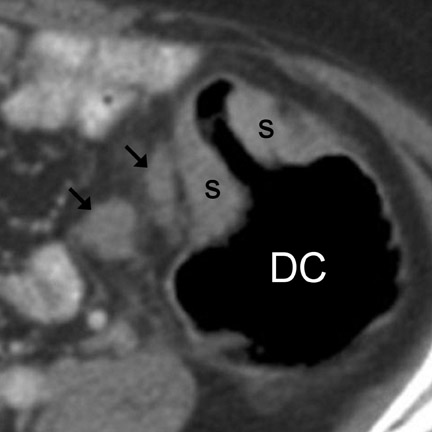
Sites of involvement- Gastrointestinal stromal tumor occurs most frequently in the stomach (70%), followed by small bowel (20% to 30%) (Figure 15), and anus/ rectum (7%). 11,12 Infrequently, GIST arises in the omentum or retroperitoneum. 11
Leiomyoma typically occurs in the stomach or proximal small bowel. Leiomyosarcoma occurs more frequently in the distal ileum (50%) rather than in the proximal small bowel. 13 However, even in the distal small bowel, GIST is far more frequent. 12,24
Imaging features and patterns of spread- These mesenchymal tumors commonly present on CT as bulky, heterogeneously hyperenhancing mural masses (Figure 15). Tumor necrosis, calcification, hemorrhage, ulceration, and cavitation are common. The mass may be exophytic (and spare mucosa), intraluminal, or both. 2,11 Liver metastases are common and frequently hypervascular. 20 Hence, arterial-phase imaging of the primary tumor and of the liver should be performed if possible. Differential considerations include adenocarcinoma, NHL, metastasis, and diverticulitis with abscess formation. The hypervascularity of the primary GIST and the metastases, if present, suggests the correct diagnosis (Figure 14).
Other mesenchymal tumors
Gastrointestinal lipomas occur most often in the ileum and colon. 2,8,12,24,25 On CT, identification of a well-defined fat-attenuating mass is diagnostic. 2,8 This benign tumor is usually an incidental, asymptomatic lesion. Rarely, a lipoma may serve as the lead point for an intussusception. 12,24 Other mesenchymal tumors, including liposarcoma and nerve sheath tumors, are rare.
Miscellaneous primary tumors
Most appendiceal mucoceles are caused by chronic infiammation (eg, chronic appendicitis) that incites mucosal hyperplasia and focal obstruction. Neoplasia (mucinous cystadenoma or cystadeno-carcinoma) is a less frequent cause. 19 On CT, distension of a fiuid-filled (high-attenuation) appendix is apparent. Pseudomyxoma peritonei, a complication of mucocele rupture, may be the only indication of the primary pathology.
Metastases to the GI tract
Metastases to the bowel may occur via hematogenous dissemination (melanoma, lung, breast, and Kaposi sarcoma), peritoneal seeding (ovary, GI primaries), or direct invasion (pancreatic and GI primaries). Hematogenous metastases typically arise along the antimesenteric border. 8,13 Bowel metastases from peritoneal seeding form along the mesenteric border. 8,13 CT findings include rounded bowel-wall masses with luminal protrusion (Figure 16). Metastases (especially breast metastases to the stomach 24 ) may cause diffuse submucosal infiltration, circumferential bowel-wall thickening, and poor distensibility, indistinguishable from primary adenocarcinoma. An unusual but important variant is a cavitating metastasis. This morphology is most common with metastatic melanoma (Figure 3) 24 and may mimic NHL, adenocarcinoma, GIST, or diverticular abscess. Complications of bowel metastases include intussusception, obstruction, and perforation.
Conclusion
Several CT imaging features of GI tumors assist in differentiation of benignity from malignancy and aid in determination of tumor etiology. Location, morphology, specific signs, and associated findings all play essential roles. Complications of both benign and malignant pathology are frequent and must be diagnosed. CT is an optimal modality to accomplish these tasks. However, proper CT technique, including the directed use of both oral and intravenous contrast material, is essential for adequate visualization and interpretation of pathology.
Related Articles
Citation
CT evaluation of gastrointestinal tumors: Imaging techniques and key diagnostic findings. Appl Radiol.
July 5, 2005