Contrast-enhanced Mammography: Successful Clinical Experience
Images
Background
There have been many recent advancements in breast imaging. In particular, screening tomosynthesis has been shown to improve cancer detection while decreasing recall rates. The use of synthesized 2D images, calculated from the tomosynthesis datasets, allows similar improvements to cancer detection while lowering the radiation dose of 2D/3D combination imaging to levels of 2D mammography. The advent of tomosynthesis-guided biopsies allows us to completely evaluate lesions detected on these new technologies.
As impressive as these recent advancements are, their gains are mainly in the area of increasing the sensitivity and specificity of screening exams for the detection of breast cancer detection. As we know, functional imaging yielding data about the physiologic activity of a lesion (such as that given by dynamic contrast enhanced breast MRI or PET imaging) has historically been unavailable in the mammographic modality. This has been particularly frustrating, given that mammography is the only modality that frequently offers radiologists multiple prior examinations by which to establish stability of findings—which often obviates the need for further work up or biopsy of lesions.
However, things are changing. Contrast mammography, which uses standard iodinated contrast agents, can now be used in conjunction with mammography equipment to answer physiologic questions about a breast lesion that breast MRI has historically answered. Published studies have shown contrast mammography to have equal or near equal sensitivity to MRI, but with higher specificity. In 2013, Hologic received clearance to market contrast-enhanced 2D mammography (CE2D) on its Selenia® Dimensions® system. This system, which is primarily a software upgrade to the company’s Dimensions unit, allows one to perform contrast procedures at the point of diagnostic evaluation, and does so with shorter procedure times, easier access, and lower costs as compared to breast MRI.
The exam can generate a 2D contrast image or a 2D contrast image combined and co-registered to a tomosynthesis dataset. In both cases the procedure creates both a morphological image (standard 2D and/or tomosynthesis image) and the functional image (contrast 2D) for review. We perform both types of imaging in our practice, depending on the case.
About Our Practice
We are a small breast clinic located in Southern Kentucky that serves 8,000 patients per year with both screening and diagnostic imaging services. In our rural geographical area, MR breast imaging has historically been challenging because there is no immediate access to high-quality breast MRI. When a facility in our area did upgrade to offer breast MRI, the facility did not offer MRI-guided biopsy, which meant that patients with MRI-detected BI-RADS 4 lesions were directed to an out-of-state facility to undergo repeat MRI and, if indicated at that point, MRI-guided biopsy. We wanted to offer accessible, accurate, and affordable services to our patients in need of contrast-enhanced imaging, and we needed the capacity to biopsy suspicious lesions that we discovered.
Our challenge, like most practices, is providing value-based care. We began using CE2D in the September of 2014 in order to expand our range of services and offer better, cost-effective care to patients. Since then, we have used CE2D in a variety of clinical applications that historically would have been evaluated with MRI. Specifically, we have used CE2D for the evaluation of patient-reported palpable regions of concern that were radiographically normal on diagnostic work-up, patients with pacemakers, patients with breast cancer to evaluate for extent of disease, radiology pathology discordance, claustrophobia, the uninsured, the underinsured and those with no access to transportation to outside facilities with MRI. It has been remarkably clinically effective to use one single modality to perform digital mammography, breast tomosynthesis, tomosynthesis-guided biopsy, and contrast imaging.
How It Works
The procedure can be broken down into two steps: contrast administration and imaging. The contrast agent is a standard non-ionic CT contrast agent that is delivered intravenously to a patient using a power injector. After waiting two minutes for the contrast agent to distribute into the breast, CE2D imaging proceeds. One can image either one or both breasts in any projection desired. For each projection, the system takes 2 separate but nearly simultaneous exposures, the first image is a standard 2D mammogram and the second a high kV mammogram. These images are subtracted automatically, resulting in a CE2D image that highlights any focal areas of enhancement. If desired, the tomosynthesis series can be acquired as part of the contrast imaging protocol. The tomo and the CE2D images are co-registered to the FFDM and can be used for biopsy guidance using the Affirm® breast biopsy system on the same gantry.
The entire procedure takes less than 10 minutes and is broken out as follows. In our experience:
- Injection of iodine. The patient is seated.
- After 2 minutes, we begin positioning the patient standing.
- We have an approximately 6-minute window where the contrast agent can be well visualized. In that time we can easily perform CC and MLO images of both breasts, and if desired, additional projections to show enhancement to best advantage.
Use of CE2D in Clinical Practice
We find CE2D is useful for a variety of circumstances. Specifically, we have used CE2D to evaluate discordant radiographic and pathologic findings.
CE2D for Discordant Findings
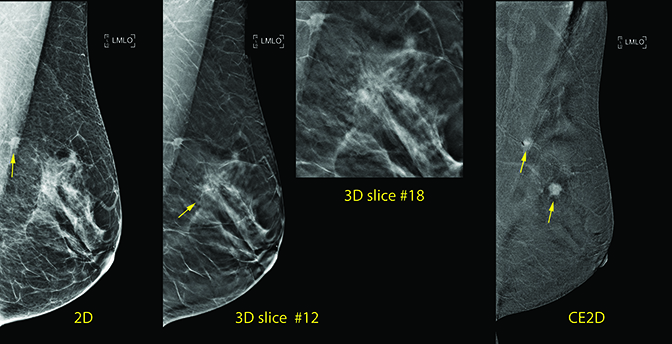
CASE 1: 52-year-old female who presented for screening, which demonstrated a 0.8 cm spiculated mass in the left axillary tail.
Imaging Results
- Screening mammogram demonstrates a 0.8 cm spiculated mass in the left axillary tail, confirmed as suspicious on tomosynthesis. Ultrasound (US) illustrates this as a 0.6 cm irregular isoechoic mass.
- Tomosynthesis also demonstrates a 0.8 cm well circumscribed mass at the 2:00 axis 2 cm from the nipple. US reveals a well-circumscribed 0.6 cm avascular hypoechoic mass with some acoustic enhancement.
- Additionally, adjacent to the mass, tomosynthesis reveals a focal area of possible distortion, without definite US correlate which therefore cannot be biopsied under sonographic guidance.
Histopathology
Ultrasound-guided biopsy of the spiculated lesion in the left axillary tail was performed and post-procedural mammogram demonstrated accurate placement of the clip within the mass. However, pathology results were discordant, reporting only fibroadipose tissue. Stereotactic biopsy was planned for the discordant lesion in the axillary tail and the distortion seen on tomosynthesis.
CE2D Imaging
Prior to tomosynthesis-guided biopsy, CE2D was performed showing suspicious intense contrast enhancement of the spiculated mass in the left axillary tail and suspicious enhancement of a 1 cm mass within the dense band of tissue at the 2:00 axis of the left breast.
The enhancing mass was cross-localized from the 2D image of the contrast exam to the 2D/Tomosynthesis exam, documenting that the distortion seen on tomosynthesis corresponded to the enhancing mass seen on CE2D. The ability to directly trace a focus of enhancement to its mammographic correlate without crossing modalities increases confidence that the etiology of enhancement is understood, and the stability of the mammographic correlate can then be directly ascertained.
Tomosynthesis-guided biopsy of the spiculated mass in the axillary tail and the distortion at the 2:00 axis of the left breast was performed. Pathology results of the spiculated lesion reported a columnar change and ductal epithelial hyperplasia. Pathology of the distortion reported a ruptured cyst with surrounding fibrosis with sclerosing adenosis with no evidence of atypia or carcinoma.
Radiographically, these were discordant, so surgical excision of both areas was performed. Surgical excision pathology demonstrated invasive carcinoma with tubular features for both lesions.
CE2D for MRI-contraindicated Patients
We have also found CE2D useful for patients in which MRI is not an option, such as those with generous body habitus, pacemaker placements, and those not amenable to MRI secondary to claustrophobia, lack of insurance coverage, or high deductible costs.
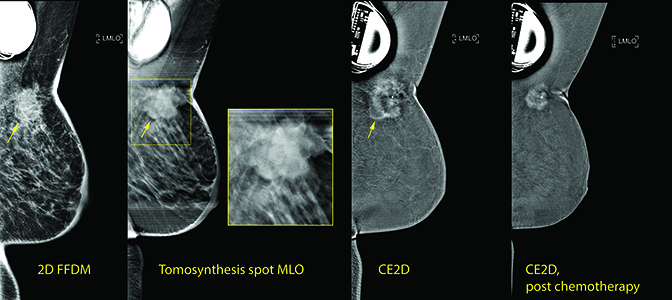
CASE 2: 50 year-old female, presenting with an area of irritation underlying the skin of her left breast.
The patient assumed the irritation was related to her pacemaker and waited for the irritation to resolve. Over 6 months, the pin-sized area of irritation developed into a 4 cm erythematous and indurated mass-like thickening overlying her pacemaker.
Imaging Results
Diagnostic evaluation with 2D/tomosynthesis demonstrated a 4 cm spiculated mass at the 1:00 axis of the left breast with associated skin thickening and skin retraction. Ultrasound showed a 3.5 cm solid irregular mass at the 1:00 axis, with the left axilla containing abnor-mal lymph nodes lacking fatty hila.
Histopathology
Ultrasound-guided biopsy of the spiculated mass and of one axillary lymph node was performed. Pathology results showed invasive ductal carcinoma within the breast mass; and metastatic ductal carcinoma involving the biopsied axillary lymph node. The patient was referred to oncology for neoadjuvant chemotherapy.
CE2D Imaging
Because the transcutaneous pacemaker negated the option of pre-treatment and post-treatment MRI to evaluate response to treatment, and PET was de- clined by insurance, CE2D was performed.
CE2D prior to neoadjuvant therapy showed strong enhancement of the mass at the 1:00 axis of the left breast. The 3.4 × 3.4 cm mass can only be seen on the MLO view secondary to fixation to the chest wall.
Following neoadjuvant chemotherapy, the CE2D exam shows strong enhancement of the mass at the 1:00 axis of the left breast, with an interval decrease in the size of the mass, now measuring 1.7 × 1.7 cm. Interval decrease in skin thickening of the right breast also seen and the mass can now be pulled into view on XCC imaging.
CE2D for Measuring Extent of Disease in Very Dense Breasts and for Evaluating Response to Neoadjuvant Chemotherapy
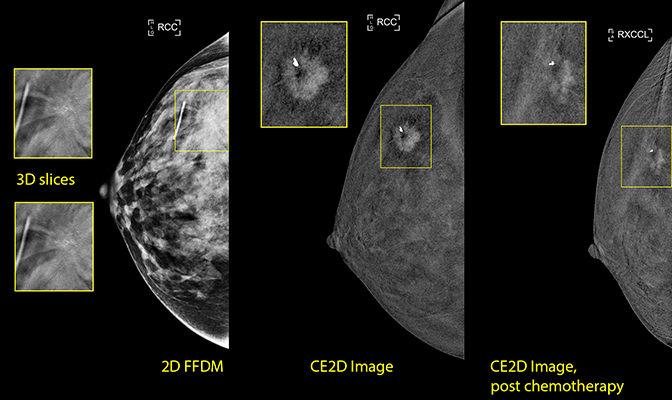
CASE 3: 49 year-old female presenting with a palpable mass with skin dimpling in the right breast.
Imaging Results
This patient underwent diagnostic 2D/tomosynthesis, which revealed a spiculated mass with associated calcifications at the 11:00 axis of the right breast. Prominent lymph nodes were also noted in the axilla. Because the mass was embedded within very dense parenchymal tissue, the extent of disease was not easily measured by standard mammography.
Ultrasound demonstrated a 1.5 cm hypoechoic mass at the 11:00 axis 4 cm from the nipple corresponding to the spiculated mass seen mammographically. Ultrasound-guided biopsy of the mass and biopsy of a prominent lymph node was performed. A clip was placed within the mass, and follow-up mammography demonstrated accurate clip placement.
Histopathology
Pathology results demonstrated invasive ductal carcinoma within the mass, as well as metastatic invasive ductal carcinoma involving the biopsied lymph node. The patient was referred for oncological evaluation and neoadjuvant chemotherapy.
CE2D Imaging
Prior to initiation of neoadjuvant chemotherapy, CE2D was performed, confirming the presence of a 1.5 cm × 1.3 cm enhancing spiculated mass at the 11:00 axis of the right breast.
After the start of neoadjuvant therapy, repeat CE2D was performed to evaluate response. The enhancing mass showed an interval decrease in size, demonstrating a partial response to neoadjuvant chemotherapy.
The patient then underwent bilateral mastectomy with right axillary lymph node dissection. Surgical pathology following mastectomy demonstrated the residual tumor within the upper outer quadrant of the right breast measured a maximum of 1 cm, concordant with the 1 cm residual enhancing lesion seen on postneoadjuvant contrast mammography.
Conclusion
We have found contrast mammography to be an excellent adjunct to our practice. In our preliminary experience, CE2D has been a clinically effective, efficient, and inexpensive tool for evaluating breast tissue and breast cancer. The advancements in this technology have allowed us to utilize one machine for screening, diagnostic evaluation, biopsy, and response to treatment. The cost effectiveness of this technology has been an excellent fit for our practice.
References
- Friedewald SM, Rafferty EA, Rose SL, et. al. Breast Cancer Screening Using Tomosynthesis in Combination with Digital Mammography. JAMA 2014;311(24):2499-2507.
- Skaane P, Bandos AI, Eben EB, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology, 2014 Jun;271(3):655-63.
- Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Diagn Interv Imaging. 2014 Mar;95(3):351-2.
- Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013:266(3):743-51.
