Congenital Venous Malformation
Images
Case Summary
A child presented with left lateral thigh pain. The patient described the pain as progressing in severity and frequency for 17 months and alleviated with increased use of nonsteroidal anti-inflammatory medications.
Image Findings
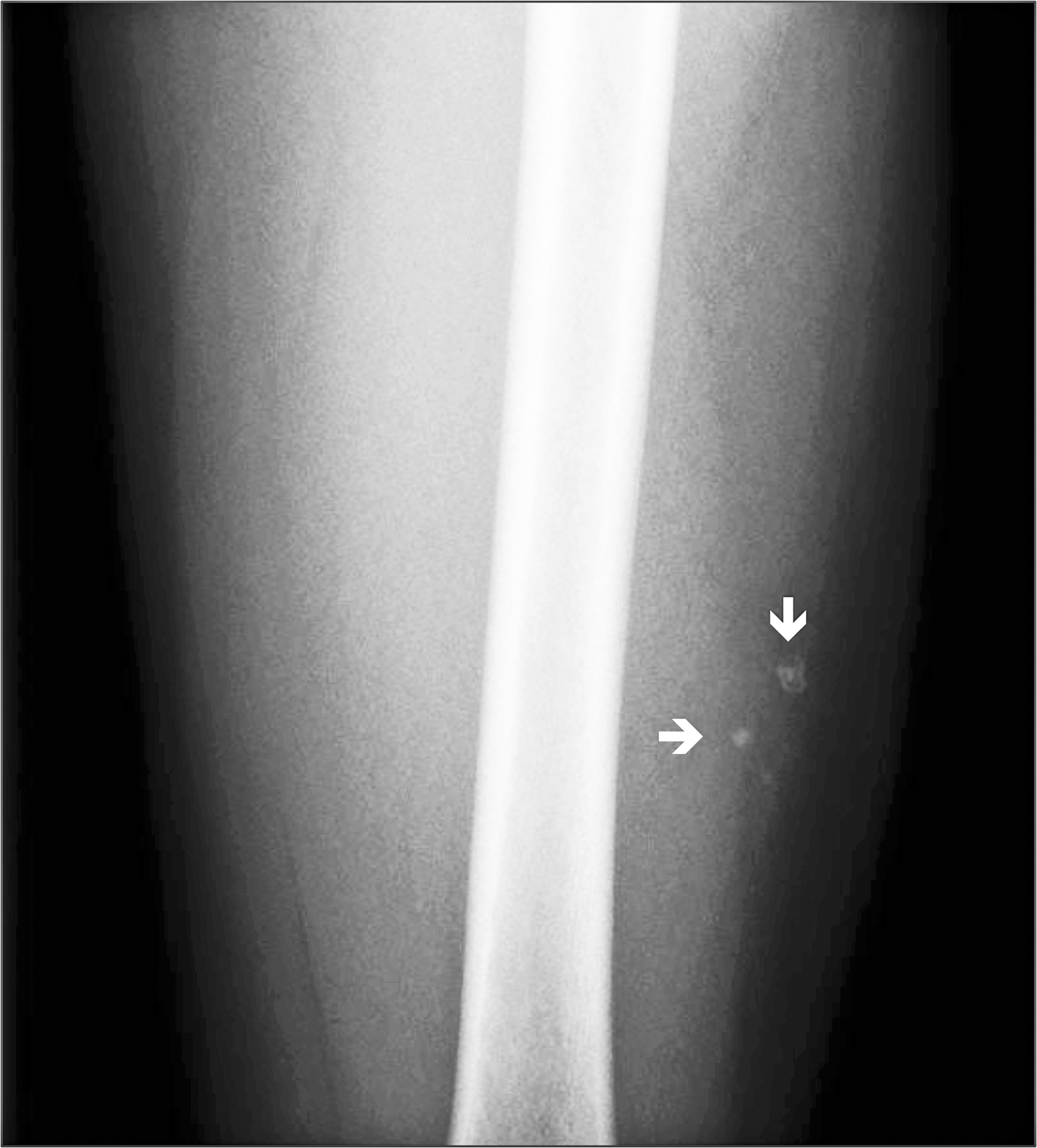
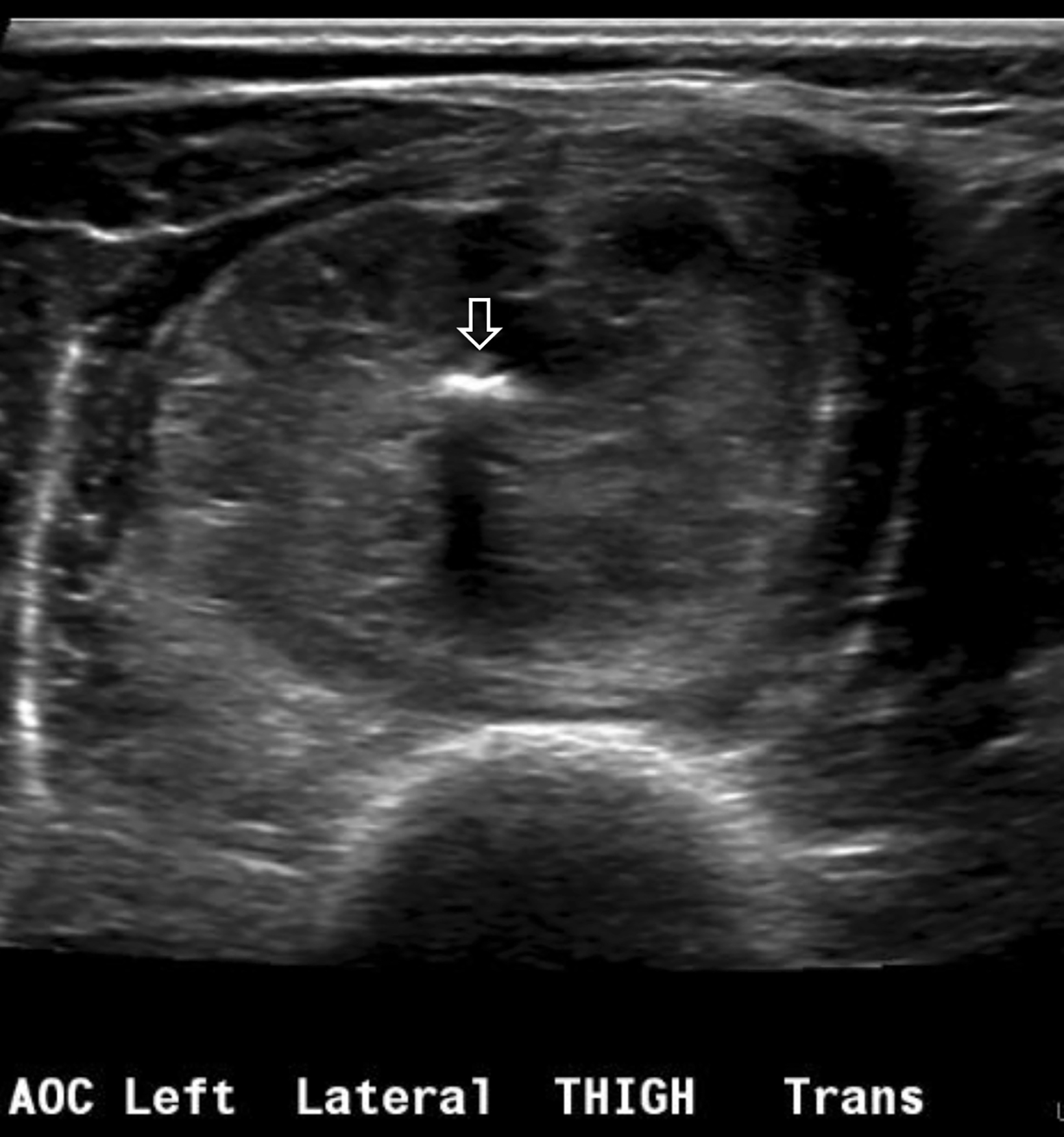
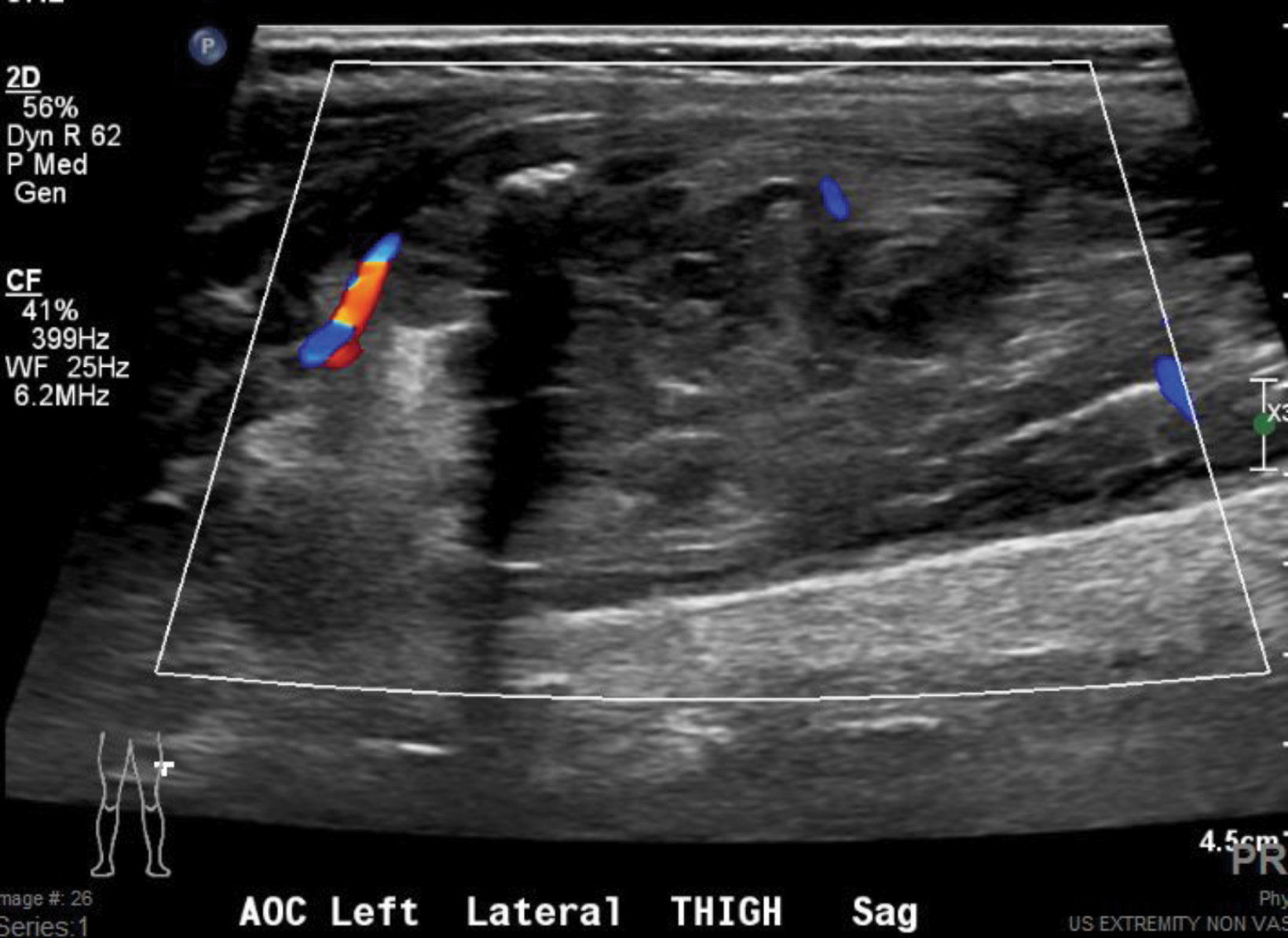
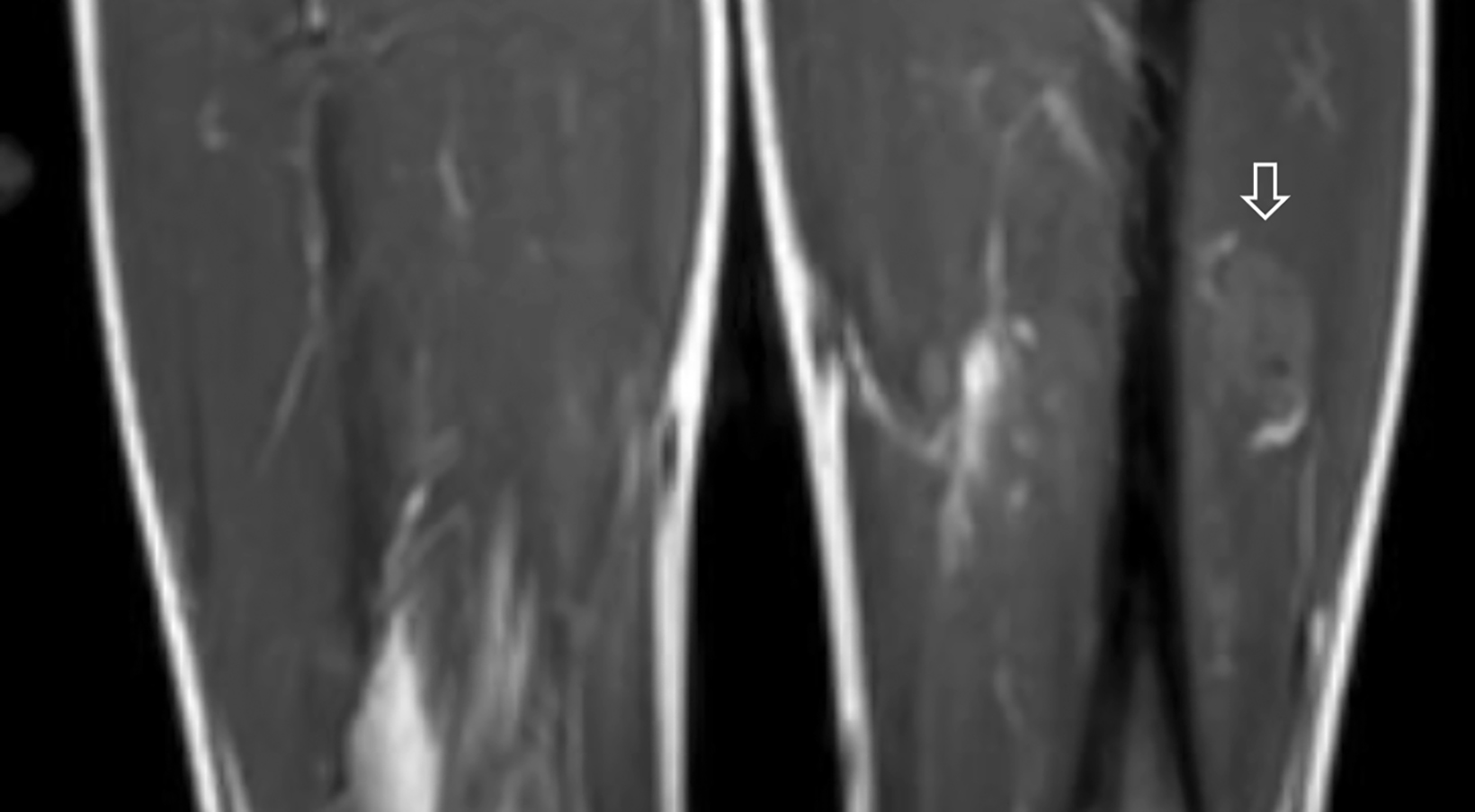
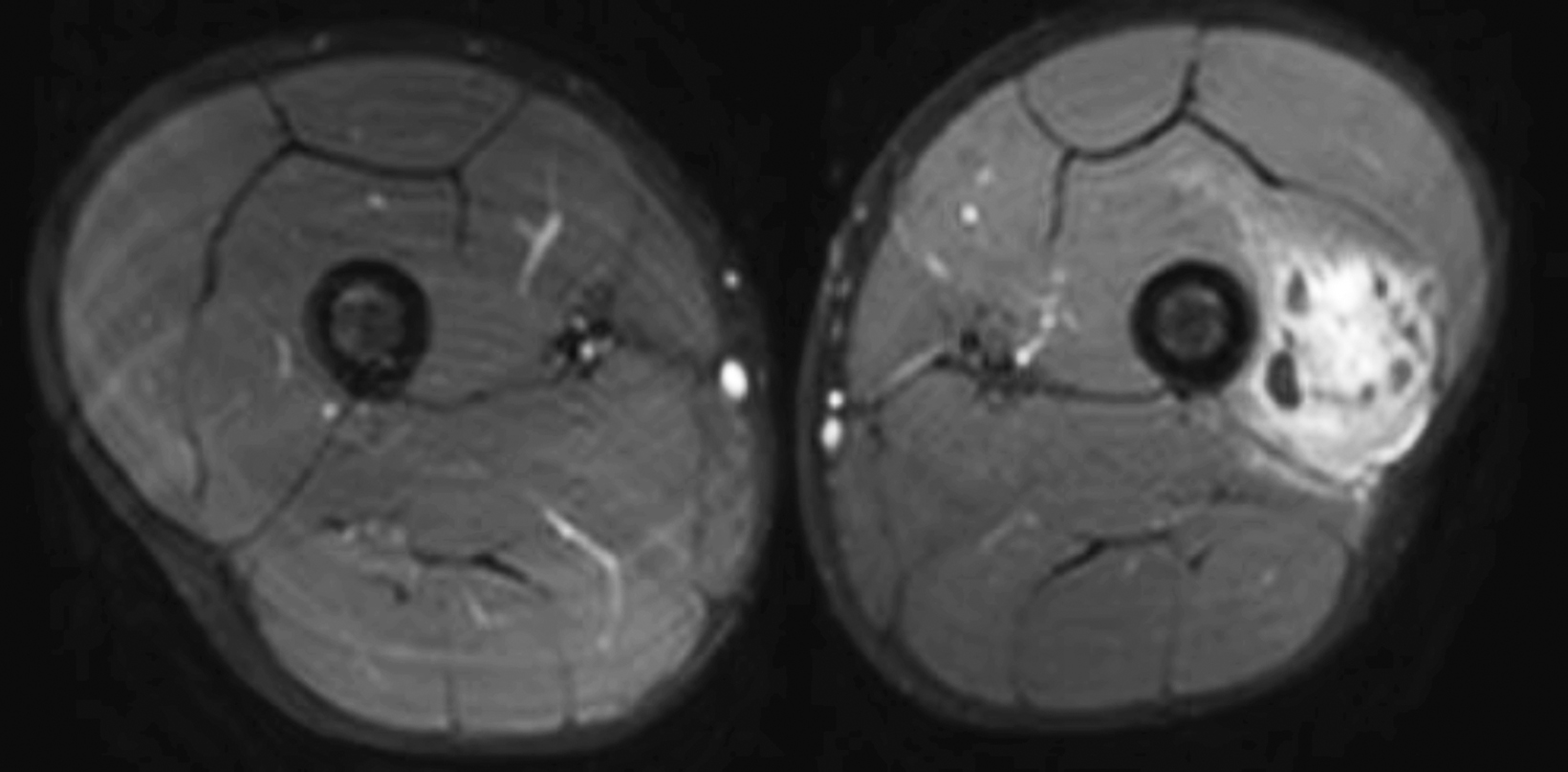
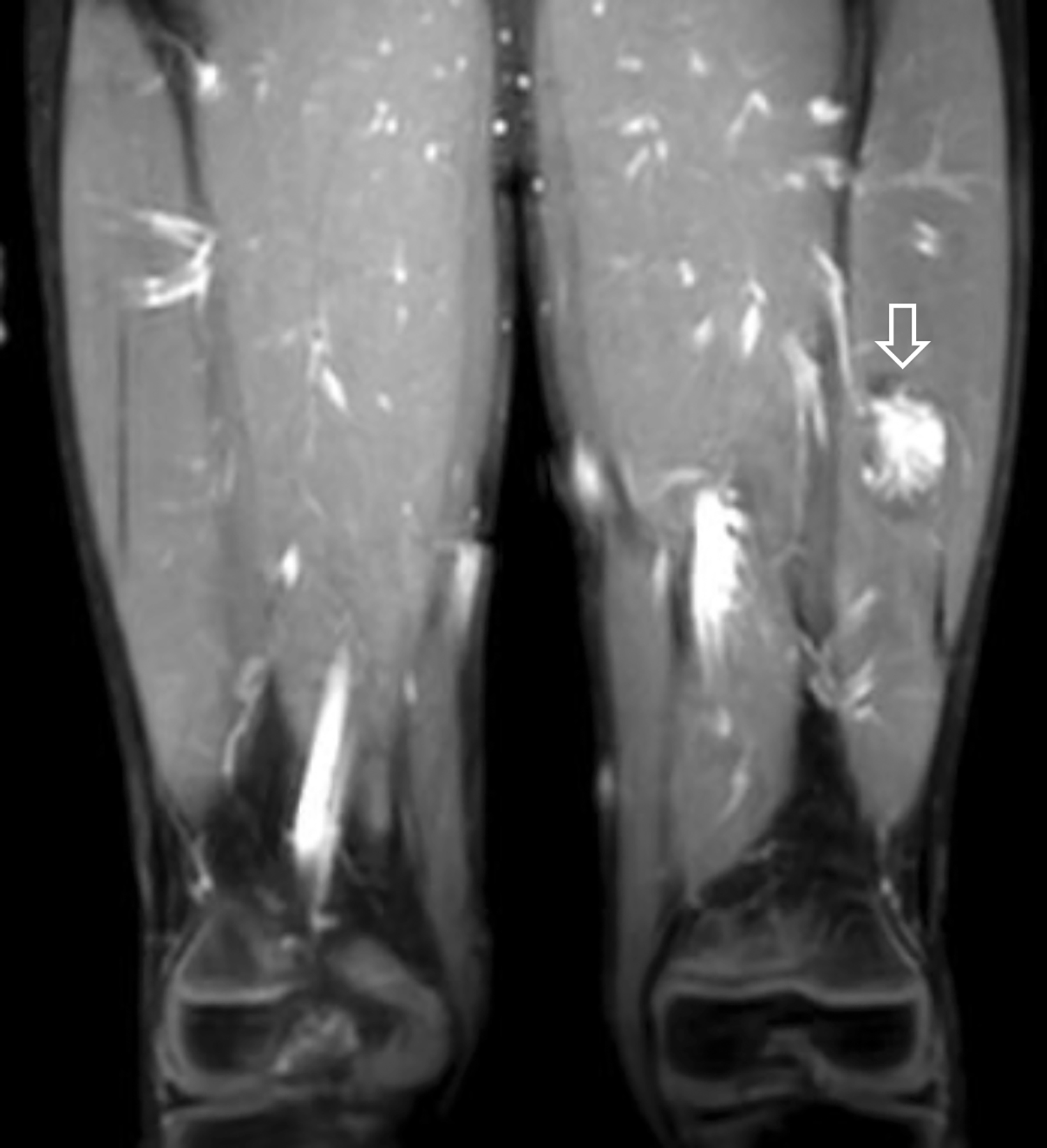
A radiograph of the left femur showed a cluster of small calcifications within the lateral soft tissues typical of phleboliths (Figure 1). Focused grayscale and color Doppler ultrasound (US) in the region of the soft tissue calcifications showed a 4.6 × 2.2 × 2.9 cm, heterogeneous, soft-tissue mass with an echogenic focus with posterior acoustic shadowing and internal blood flow (Figure 2). For further evaluation, left lower-extremity MR imaging was recommended. The MRI revealed a lobulated, complex mass within the vastus intermedius muscle with heterogeneous signal and contrast enhancement with nonenhancing foci, indicative of the phleboliths (Figure 3). An ultrasound-guided core-needle biopsy was performed; it confirmed diagnosis of a partially thrombosed venous malformation. The lesion was treated with sclerotherapy using absolute ethanol as the sclerosing agent.
Diagnosis
Congenital venous malformation. The differential diagnosis includes other slow-flow vascular malformations, specifically microcystic and macrocystic lymphatic malformations.
Discussion
The International Society for the Study of Vascular Anomalies classification of vascular anomalies categorizes vascular malformations according to the prominent abnormal vessel type. These types include the following: venous malformation (VM), lymphatic malformation (LM), capillary malformation (CM), arteriovenous malformation (AVM), and arteriovenous fistulae (AVF).1
Venous malformations are the most common type of congenital vascular malformation; they have an incidence of about 1-2 per 10,000 and a prevalence of 1%. A VM is congenital, composed of abnormally formed veins that assume abnormal morphologies. The venous walls are abnormally thin with little smooth muscle, predisposing the vessel to dilatation.2 Patients can have one or more VMs. Importantly, like all vascular malformations, VMs do not spontaneously resolve and they may recur. Clinically, VMs usually present at birth and grow proportionate to the patient’s body size. However, some VMs are not evident until later in life especially those that are deep in the body. VMs are usually not painful, although some patients experience achy discomfort, especially later in the day, resulting from stasis of blood and venous engorgement or VM partial thrombosis. A significant increase in pain and swelling can occur owing to an increase in mass effect or local hemorrhage. When VMs are superficial, they appear bluish, are compressible, and expand following compression release (in contrast to lymphatic malformations).
VMs may be familial in 1-2% of cases and are cutaneomucosal in distribution. Familial VMs are associated with a mutation of the protein receptor tyrosine kinase (TIE-2 gene) mapped to the 9p21-22 gene. VM subtypes occur in about 10% of individuals diagnosed with a VM. The clinical features and bodily distribution vary with specific subtype. Subtypes include:
- Glomuvenous malformations (GVM). GVMs occur sporadically or can be transmitted in an autosomal dominant fashion. The lesions appear as non-compressible, hyperkeratotic dark blue lesions, most often distributed superficially. Glomus cells are smooth muscle cells thought to regulate blood flow. In GVMs, these cells are abnormal and may form multiple rows within the vascular space. GVMs may be solitary or multiple (10% of cases). The solitary type is often more painful, a feature that can differentiate it from the more common congenital VM. GVMs are congenital in at least 65% of patients due to a mutation in the glomulin gene (1p21).
- Blue Rubber Blue Nevus Syndrome lesions. (BRBN)/Bean Syndrome is a sporadic disorder characterized by multiple small, dark blue, rubbery lesions most often distributed on the skin and in the musculoskeletal and gastrointestinal systems. Histologically, these lesions have deficient smooth muscle cells and dysplastic venous channels.Maffucci syndrome lesions. Maffucci syndrome is a sporadic genetic disorder characterized by multiple enchondromas and spindle cell hemangiomas. The spindle cell hemangiomas are most often found asymmetrically on palms, soles, and extremities. Long-term effects may include malignant degeneration of the enchondromas to chondrosarcomas and disfigurement resulting from the bone and vascular lesions.
- Cerebral Cavernous Malformation (CCM). CCM is a familial disorder associated with multiple VMs in the brain that often expand and bleed. About 10% of these patients also develop VMs of the skin. The CCMs are characterized histologically by clusters of hyalinized capillaries surrounded by gliosis and hemosiderin deposition. CCMs are most often found in the supratentorial brain and can be associated with developmental venous anomalies. MRI signal will vary depending on the presence and age of blood products.
Imaging is not often necessary to diagnose cutaneous VMs, but it is essential for evaluating deep lesions. However, diagnostic imaging and treatment planning are required when localized VMs present with pain, swelling, neurologic symptoms, or other forms of disfigurement and functional impairment or planning for image-guided sclerotherapy. Radiographs occasionally may be useful in diagnosing deep VMs if phleboliths or dystrophic calcifications are identified. Bony changes may be found in up to a third of patients with VMs.3 The presence of phleboliths can distinguish VMs from other types of vascular malformations; eg, lymphatic malformations do not have phleboliths. US and MRI are key to the diagnosis and differentiation of vascular malformations. Although CT may be more sensitive in identifying phleboliths, the modality is no longer used as a primary diagnostic modality owing to its ionizing radiation and lower (compared to MRI) soft-tissue resolution/differentiation.2
Doppler US is often useful for initial examination of a suspected vascular malformation. The typical diagnostic features of VMs include a compressible, soft-tissue mass made up of venous channels and a relatively small amount of soft tissue. VM lesions are hypoechoic or anechoic masses with slow intralesional blood flow. During contrast-enhanced US, enhancement may be seen in the venous phase.
MRI with gadolinium contrast enhancement is the gold standard for diagnosis. VMs typically appear as septated, lobular masses with high-intensity signal on T2 or STIR imaging and intermediate-low signal on T1 images; there is also a lack of flow voids. Importantly, no abnormal arterial flow or arterial enlargement is present. Typically, VMs do not exert mass effect on adjacent structures. Diffuse enhancement on postcontrast imaging is also indicative of VMs. Phleboliths are identified by low intensity foci on all pulse sequences.4
Treatment options include medical management, surgery, laser therapy, and sclerotherapy. Medical management includes low-dose NSAIDs for pain control and, in some cases, low molecular weight heparin to prevent localized coagulopathy.5 Compression stockings can be used for swelling in the extremities. However, these medical therapies do not treat the underlying pathology.
Image guided sclerotherapy is today’s first-line treatment for VMs.6 The goal is to cause intimal injury to endothelial cells that leads to localized, intralesional thromboses and ultimate fibrosis so that no significant blood flow occurs in the VM. Multiple sclerotherapy sessions are often needed to significantly improve the lesion; US is used to guide sclerotherapy and to monitor treatment progression.
The choice of treatment agent is often related to the preference and experience of the treating physician. Ethanol is one of the most common and effective sclerosing agents. Alternative agents include sodium tetradecyl sulfate (STS) and polidocanol, which are less effective in our experience. Bleomycin is a newer, effective option with fewer complications than absolute ethanol, especially with respect to skin necrosis. However, the therapeutic effect takes considerably longer and requires longer intervals between follow-up visits. Post-procedurally, patients are managed with analgesics and anti-inflammatory drugs.
The expected side effects of sclerotherapy include edema and inflammation; the VM and surrounding tissue commonly appear swollen and painful to touch post-therapy. Major complication rates vary with sclerosing agent.7 A retrospective study of outcomes in 153 patients with VMs reported a grade 3 (requiring plastic surgery intervention) complication rate of 2% and a grade 4 (permanent scar at injection site) complication rate of 7%.8 The most common serious complications include disseminated intravascular coagulation, nerve injury, infection, and skin necrosis. On rare occasions, local swelling can cause a compartment syndrome. However, complications such as pulmonary hypertension, pulmonary embolism, arrhythmia, and hemoglobinuria can occur if the sclerosing agent enters the systemic circulation. Because of these risks, some practices routinely obtain blood alcohol levels to monitor the patient post-sclerotherapy and use a weight-based dosage of ethanol.
Mean time to potential recurrence is 7-37 months.9 The literature consistently reports clinical improvement among most patients after sclerotherapy, although many report previous treatment for VMs. Recovery is assessed based on a combination of patient’s subjective symptoms and reduction in lesion size.
Conclusion
Venous malformations are the most common type of congenital vascular malformation and are usually not related to familial or syndromes causes. Most VMs are diagnosed clinically with imaging. The primary therapy today is minimally invasive, image-guided sclerotherapy.
References
- Monroe EJ. Brief description of ISSVA classification for radiologists. Tech Vasc Interv Radiol 2019; 22:100628.
- Steiner JE, Drolet BA. Classification of vascular anomalies: an update. Semin Intervent Radiol. 2017; 34:225-232.
- Behravesh S, Yakes W, Gupta N, et al. Venous malformations: clinical diagnosis and treatment. Cardiovasc Diagn Ther. 2016;6:557-569.
- Flors L, Leiva-Salinas C, Maged IM, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011;31:1321-1331.
- Hage AN, Chick JFB, Srinivasa RN, et al. Treatment of venous malformations: the data, where we are now, and how it is done. Tech Vasc Interv Radiol. 2018; 21:45-54.
- Pappas DC Jr., Persky MS, Berenstein A. Evaluation and treatment of head and neck venous vascular malformations. Ear Nose Throat J. 1998; 77(11): 914-16, 918-922.
- Ali S, Mitchell SE. Outcomes of venous malformation sclerotherapy: a review of study methodology and long-term results. Semin Intervent Radiol. 2017; 34:288-293.
- Gorman J, Zbarsky SJ, Courtemanche RJM, Arneja JS, Heran MKS, Courtemanche DJ. Image guided sclerotherapy for the treatment of venous malformations. CVIR Endovascular 2018; 1:2. doi:10.1186/s42155-018-0009-1
- Khaitovich B, Kalderon E, Komisar O, Eifer M, Raskin D, Rimon U. Venous malformations sclerotherapy: outcomes, patient satisfaction and predictors of treatment success. Cardiovasc Intervent Radiol. 2019;42:1695-170.
References
Citation
D N, RB T, CM S, AJ T, DJ A.Congenital Venous Malformation. Appl Radiol. 2021; (6):11-15.
November 5, 2021