Comprehensive Review of Clinical Presentation, Multimodality Imaging, and Therapeutic Strategies for Inflammatory Breast Cancer
CME for this article is available here.
Introduction
Inflammatory breast cancer (IBC) is a rare, locally advanced invasive cancer that commonly presents with skin redness (erythema) and swelling (edema). Diagnosing IBC is difficult, and prompt identification is crucial when a patient presents with a reddened or inflamed breast. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results data, the incidence of IBC was 2.76 cases per 100,000 from 1973 to 2015.1 IBC has a higher mortality rate compared with noninflammatory advanced breast cancers,1, 2 with an overall relative 5-year survival rate of approximately 40.5%, which is even lower among Black patients.1
Specific clinical criteria for diagnosing IBC include rapid onset of erythema, edema, and/or peau d’orange with a duration of less than 6 months; inflammation occupying more than one-third of the breast; and pathological confirmation of invasive carcinoma.3 The rapid onset distinguishes IBC from noninflammatory, locally advanced cancer.4 In addition to clinical diagnosis, multimodality imaging tests improve IBC detection and confirmation, with the aim of improving overall survival. Breast MRI has been found to be most beneficial for identifying primary lesions and evaluating treatment response, while18F FDG PET/CT plays a significant role in detecting distant metastases at initial diagnosis for appropriate treatment selection. Here, we offer an overview of current approaches to diagnosis and treatment of IBC.
Clinical Diagnosis
The clinical presentation of IBC arises from the diffuse and rapid obstruction of lymphatics in the breast by tumor emboli, leading to edema and hyperemia of the blood vessels in the skin.5 The most commonly used definition of IBC is based on the American Joint Committee on Cancer (AJCC) Staging System, 8th edition. This system defines IBC, stage T4d, as a clinicopathological entity characterized by diffuse erythema and edema involving approximately a third or more of the skin of the breast.6 The system requires a pathological diagnosis of invasive cancer in less than 6 months from initial symptom presentation6 ; however, while pathological identification of dermal lymphatic emboli is pathognomonic, it is not required for diagnosis. In addition, although skin erythema is a mandatory criterion for diagnosis under the staging system, it may not be present or may fluctuate or diminish over time.
The difficulty of diagnosing IBC was highlighted in an extensive external review of medical photographs and records of 270 patients with IBC across 6 sites in Egypt, Tunisia, and Morocco.7 The clinical diagnosis was based on an expert panel consensus statement. Among the cases, 76% met the consensus criteria, but only 36% adhered to the AJCC 8th edition.6, 8 Nevertheless, 86% of the cases were confirmed as IBC through photographic review adherence to the consensus statement by independent, external experts.7 An expert panel convened by the Susan G. Komen Foundation recently validated a more formal and quantitative definition of IBC that incorporates clinical, pathological, and imaging features that may improve diagnosis.9
The incidence of IBC among women with breast cancer is low, typically estimated at 2-3%.1, 10 However, according to the available references, IBC’s incidence among women presenting with breast inflammation ranges from 5-50%.11 - 13 Dabi et al proposed a diagnostic algorithm emphasizing the importance of identifying and treating IBC as an oncologic emergency. According to this algorithm, all nonlactating patients with inflammatory symptoms should undergo imaging. If malignancy is suspected but no focal mass abnormality is amenable to biopsy, a skin punch biopsy of the most involved skin should be obtained. A negative biopsy indicates the need for MRI with biopsy.11 In lactating patients with strongly suspected acute mastitis, beginning with a “test and treat” strategy is a reasonable approach. If no improvement is observed within 2 weeks of antibiotic therapy, further imaging studies should be obtained.11 At one tertiary surgical referral center, IBC accounted for 50% of cases presenting with inflammatory symptoms.12 Thus, a high index of suspicion is warranted. Patients with presumed benign mastitis that does not resolve quickly with medical therapy should undergo imaging and image-guided biopsy.
Radiological Diagnosis of IBC
Mammography
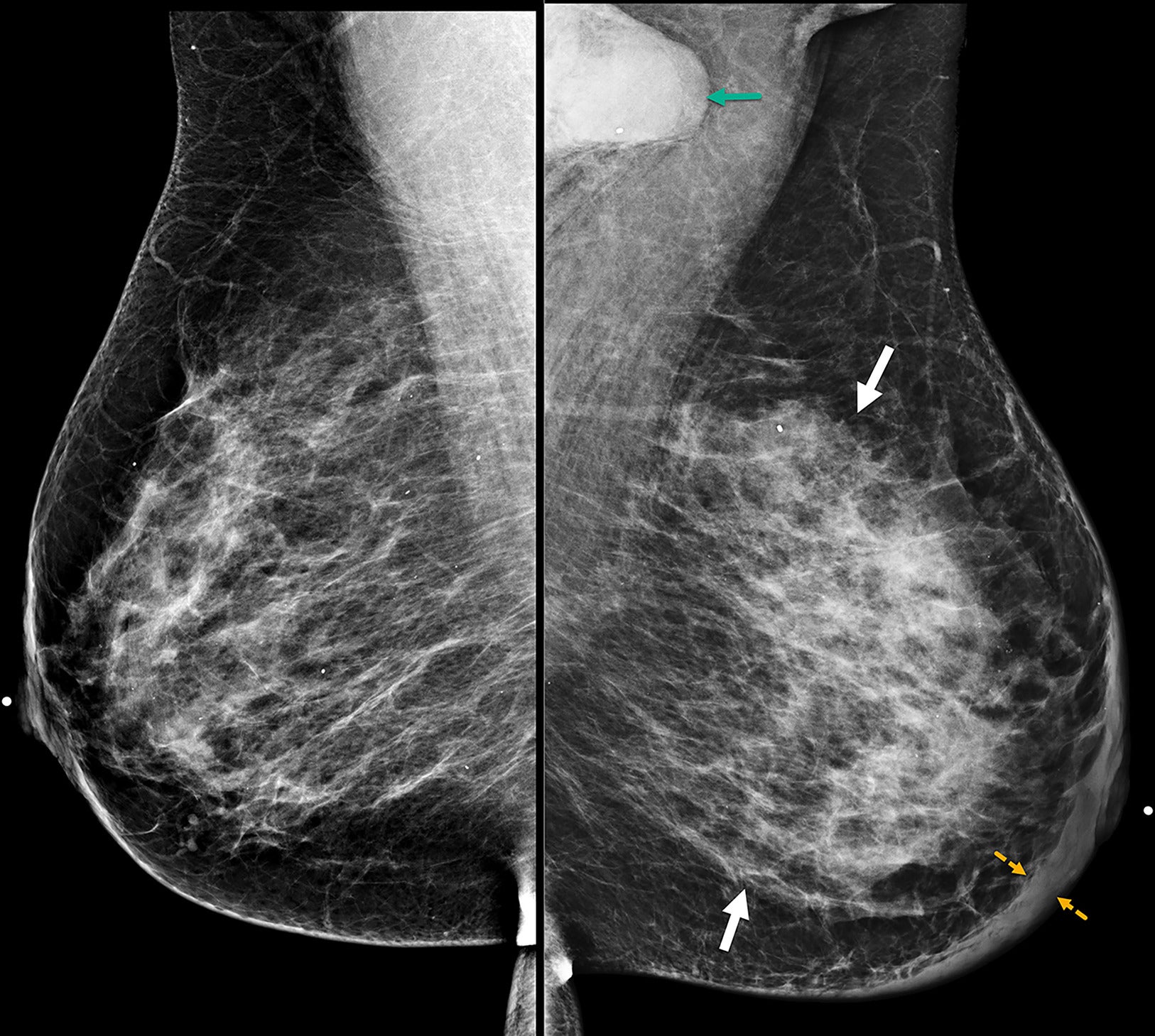
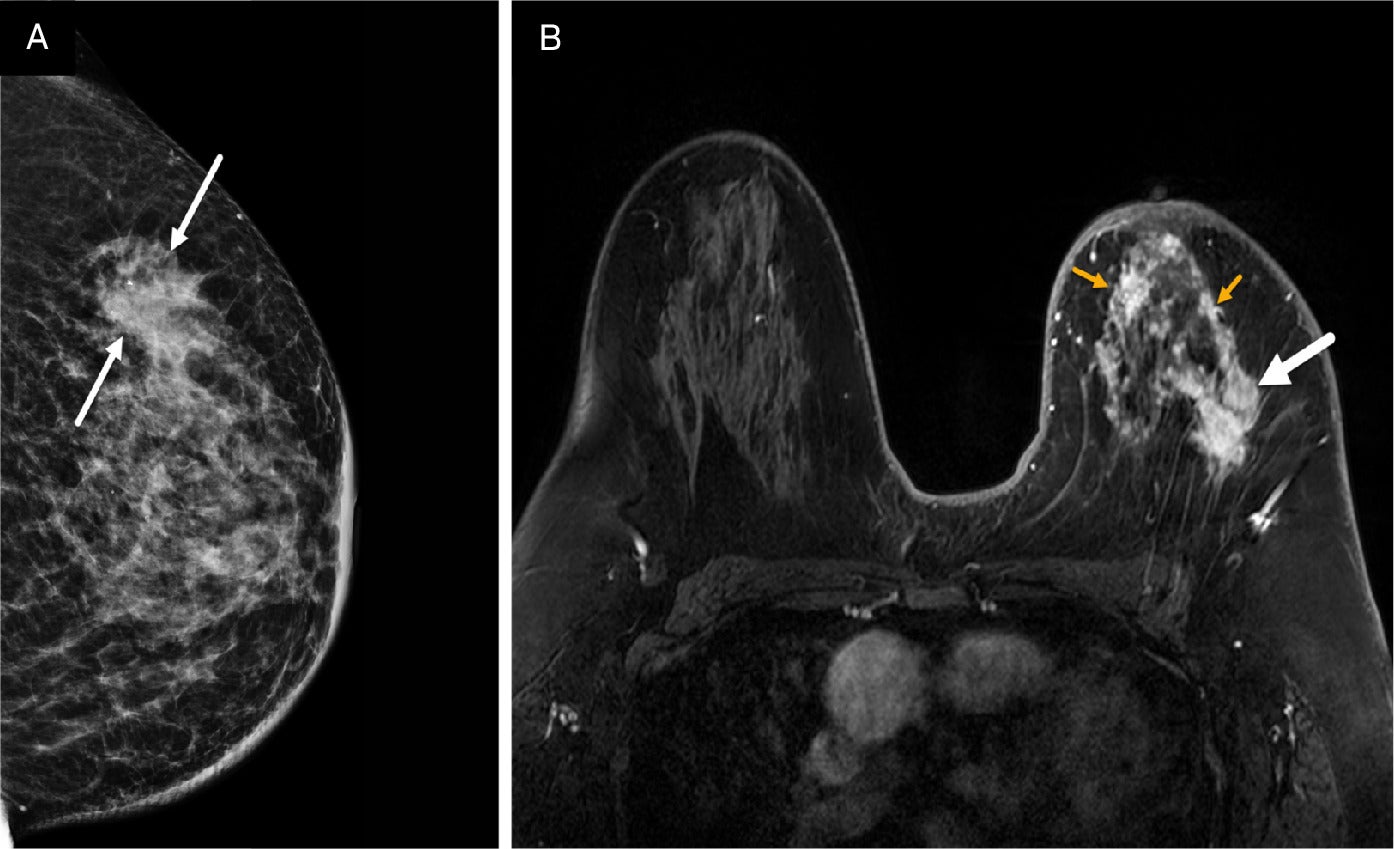
Patients with suspected IBC are often initially referred for mammography despite the modality’s limitation in detecting lesions in dense breast parenchyma. In many cases, no identifiable mass may be observed and/or the mammogram may be interpreted as normal.13 The features most associated with IBC include diffuse breast enlargement, trabecular thickening, global skin thickening, and ipsilateral axillary lymphadenopathy ( Figure 1 ).14 - 16 Skin and trabecular thickening, although nonspecific, are subtle early findings observed in 80% of IBC cases.15 - 17 Less commonly seen mammographic findings include a visible irregular mass, architectural distortion, or calcifications ( Figures 2 , 3 ).14, 17
Bilateral mediolateral oblique digital mammogram at the diagnosis of the inflammatory breast cancer case demonstrates diffuse left breast enlargement, trabecular thickening (solid white arrows), and global skin thickening up to 9 mm (dashed yellow arrows). BI-RADS Category 5: highly suggestive of malignancy. Left axillary adenopathy is partially visible on mammogram (green arrow). Breast biopsy at the 12 o’clock position reveals triple-negative invasive ductal carcinoma and ductal carcinoma in situ. Biopsy of the left axillary node was positive for malignancy.
Mediolateral oblique left digital mammogram demonstrates diffuse skin thickening (solid yellow arrow) and suspicious, fine-linear pleomorphic calcifications (double-head dashed white arrow) spanning over 8.5 cm in the upper outer quadrant. BI-RADS Category 4C: suspicious for malignancy.
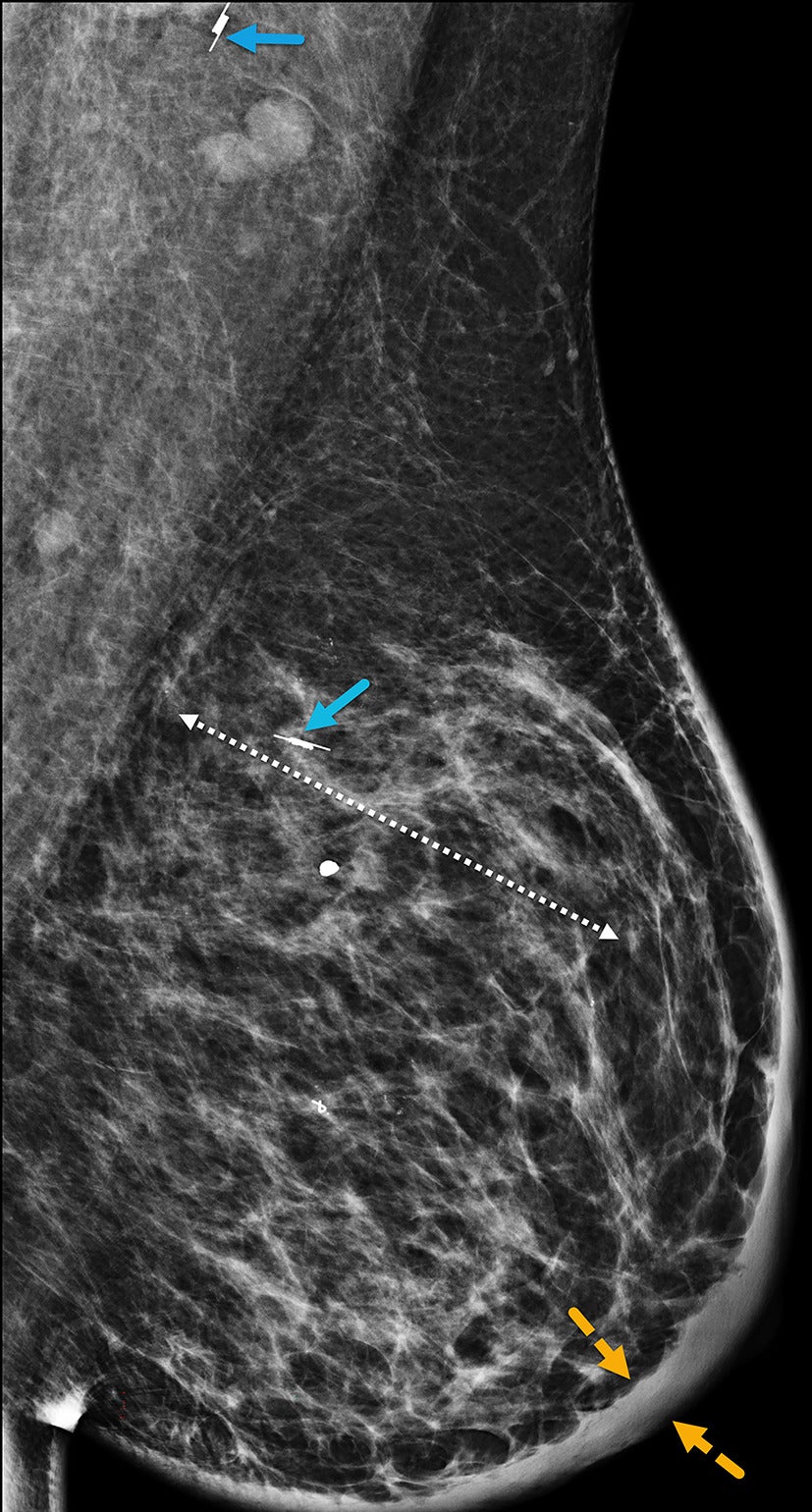
Figure 2 MLO magnification view of Figure 2 better visualizes the pleomorphic calcifications (dashed white arrows).
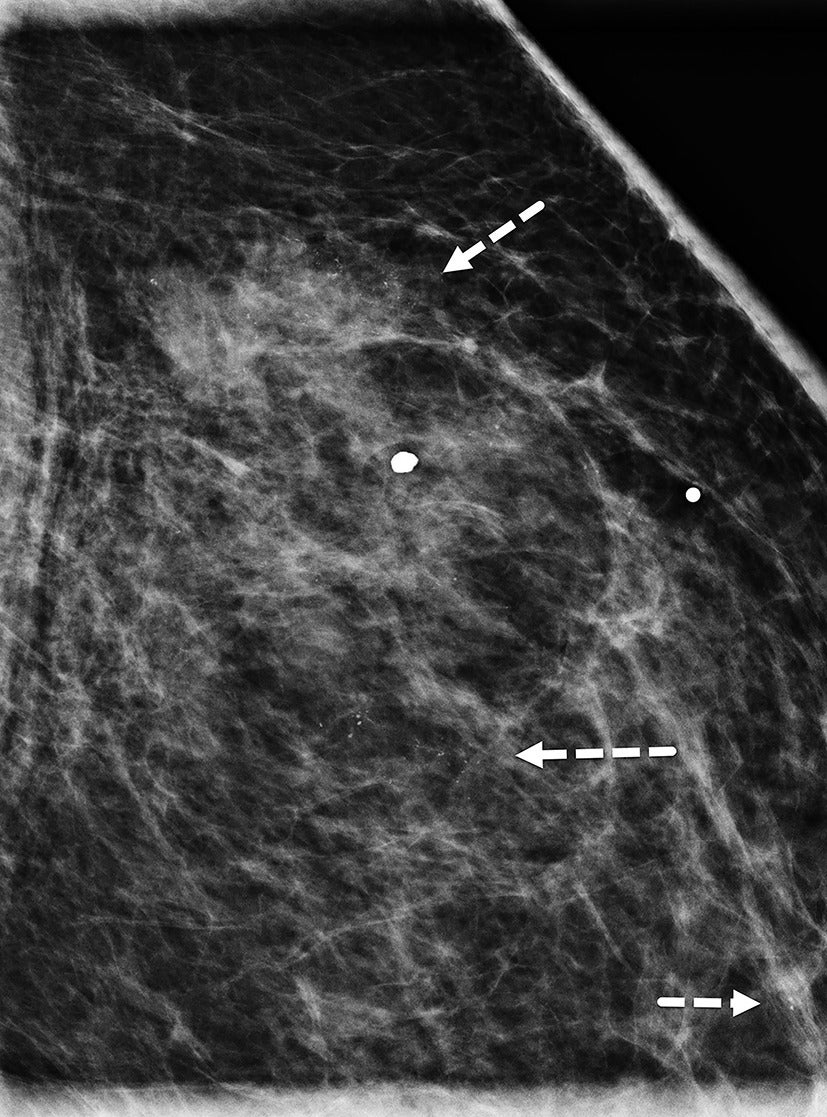
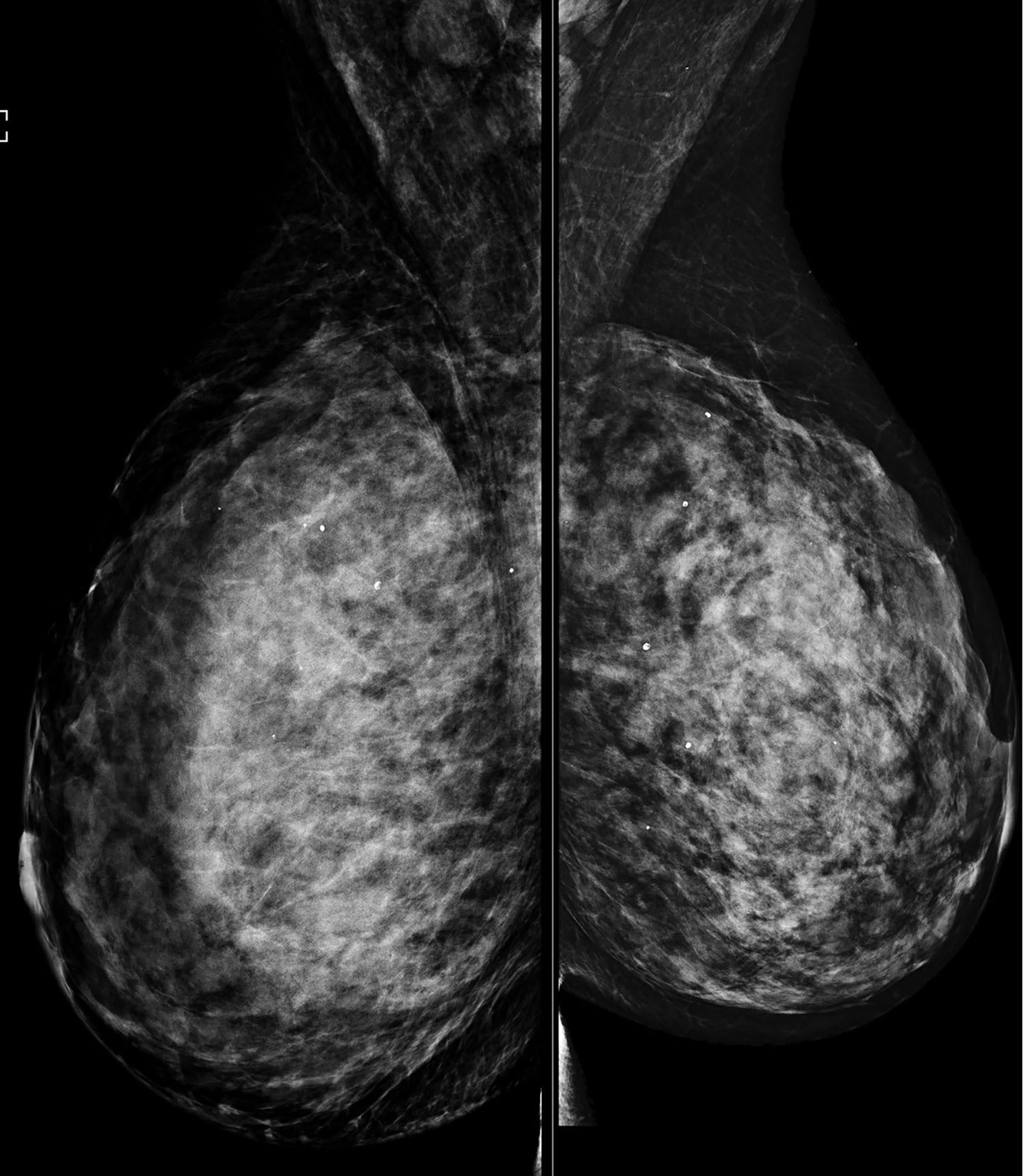
The ability of mammography to detect a primary breast lesion in patients with IBC is limited; one retrospective study found that only 20% of cases demonstrated a detectable primary lesion on mammography.15 97% of subjects in the same study had nonfatty breasts, leading the authors to suggest that the dense breast parenchyma background likely contributed to the poor visibility of lesions ( Figures 4 , 5 ).15 Another study observed that findings of skin thickening, axillary adenopathy, trabecular thickening, and nipple-areolar swelling were significantly more frequent in IBC than in non-IBC cases, while the presence of a mass was more commonly associated with non-IBC cases.18 Compared with other imaging modalities, mammography is the least sensitive to multifocal and multicentric disease in patients with IBC.15
Mediolateral oblique view shows dense breast parenchyma, which obscures underlying masses. BI-RADS Category 0: incomplete, requires additional imaging evaluation.
Axial postcontrast MRI of the same case as that in Figure 4 demonstrates multiple suspicious, enhancing masses (white arrows) throughout the dense right breast. Early phase dynamic MRI reveals tumoral enhancement from the delayed enhancement of background dense breast tissue. BI-RADS Category 5: highly suggestive of malignancy.
Ultrasound
Common sonographic features of IBC include one or more masses, skin thickening, tissue edema with lymphatic dilation, and regional lymphadenopathy.15 - 20 However, these are nonspecific features that overlap with findings seen in benign conditions such as mastitis and in other malignancies such as locally advanced breast cancer ( Figure 6 ). As a result, diagnosis with ultrasound alone can be challenging. If an index mass is identified, its common sonographic morphology includes hypoechoic mass with lobulated or irregular margins and posterior acoustic shadowing.21 - 23 Ultrasound does not reliably detect microcalcifications, for which mammography remains the most sensitive modality for identification. Architectural distortion and diffuse posterior acoustic shadowing ( Figure 7 ), often multifocal and multicentric in distribution, are observed in over 80% of cases.15, 16
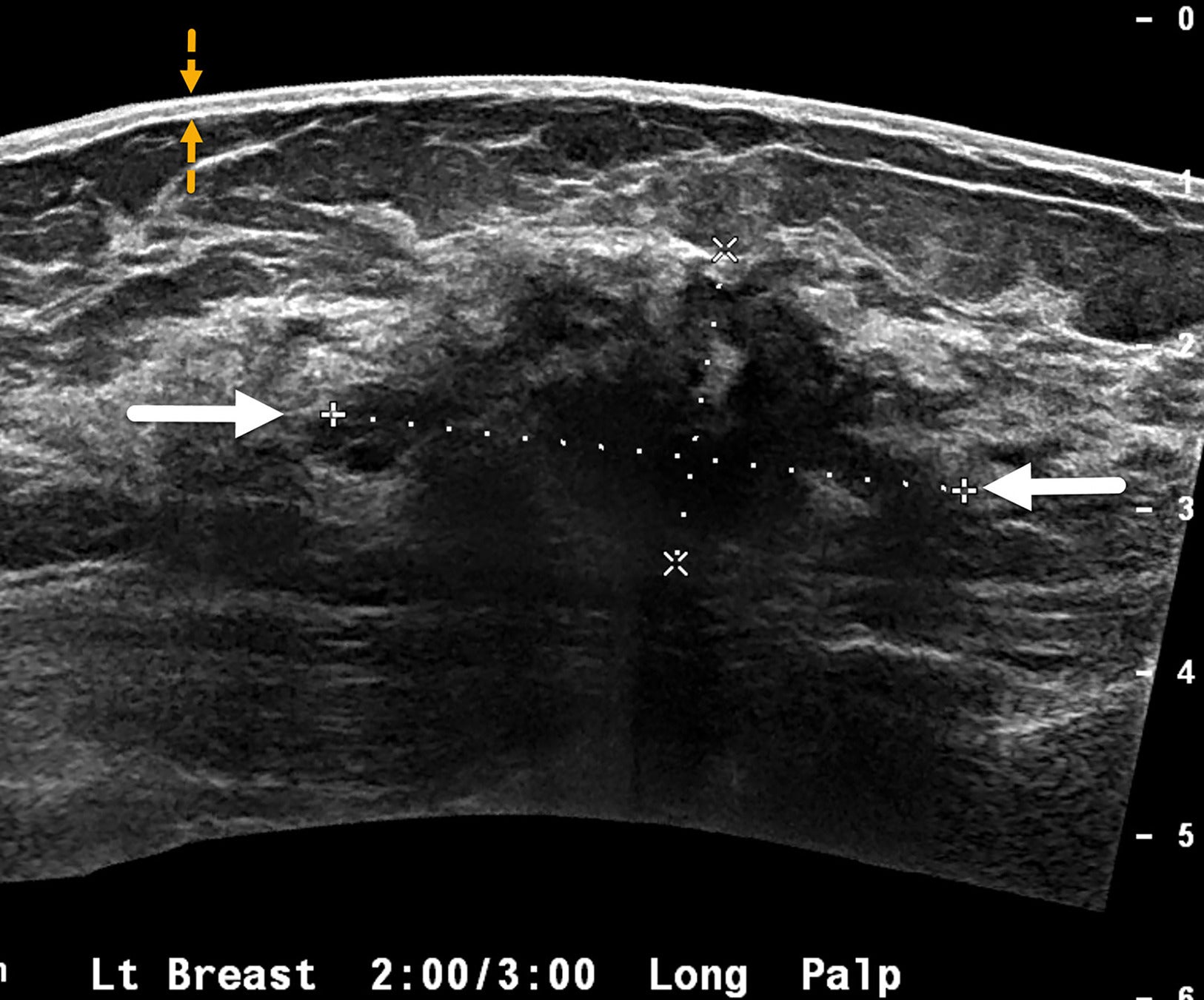
Sonogram of the left breast in a case of invasive ductal carcinoma shows a 4 cm mass with irregular margins and posterior acoustic shadowing (white arrows). BI-RADS Category 5: highly suggestive of malignancy. The skin is normal (yellow arrows), without thickening typically seen in inflammatory breast cancer.
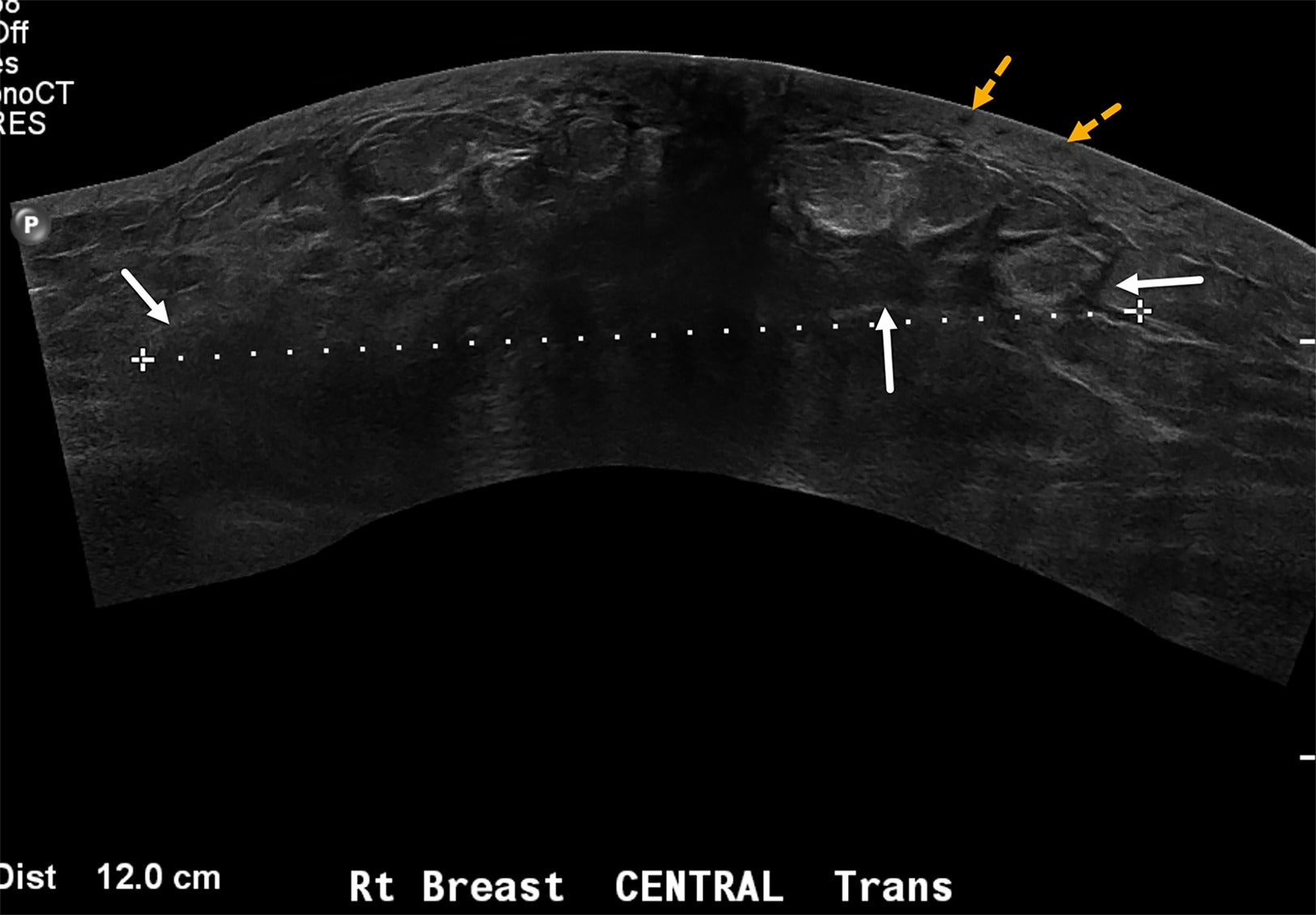
Wide FOV sonogram of the right breast in a case of newly diagnosed inflammatory breast cancer demonstrates ill-defined hypoechoic architectural distortion (solid white arrows) with diffuse posterior acoustic shadowing measuring 12 cm (dotted line). Unlike the case in Figure 6 , diffuse tumoral infiltration of the entire right breast and skin thickening—but no discrete breast masses—were detected. The thickened skin contains tiny hypoechoic lesions (yellow dotted arrows) suggestive of dermal lymphatic involvement. BI-RADS Category 4C: high suspicion for malignancy.
The inflammation seen in IBC is associated with increased vascularity of the breast lesions and surrounding parenchyma, which correlates with the erythema observed on physical examination. Diffuse skin thickening, a hallmark of IBC, can be quantified on ultrasound. Skin and breast edema are also commonly noted ( Figure 7 ). The dermal emboli and dermal lymphatic involvement characteristic of IBC may account for sonographic findings of diffuse hypoechoic and thickened skin with an indistinct dermal-subcutaneous fat interface.20, 24 Breast edema can extend into the chest wall and pleural spaces and may require multimodality imaging for complete evaluation.
MRI
MRI is superior to mammography and ultrasound in identifying index tumor masses ( Figure 8 ), which are also referred to as primary breast parenchymal lesions (BPLs).15, 19 In a study at our center, MRI successfully identified all BPLs, while ultrasound identified 95%, and mammography 80%, of lesions in patients with a clinical diagnosis of IBC.19 Owing to its high sensitivity, MRI is recommended early in the evaluation of patients with clinical suspicion for IBC, particularly when mammography and ultrasound fail to detect lesions. Additionally, MRI findings can guide biopsy procedures.
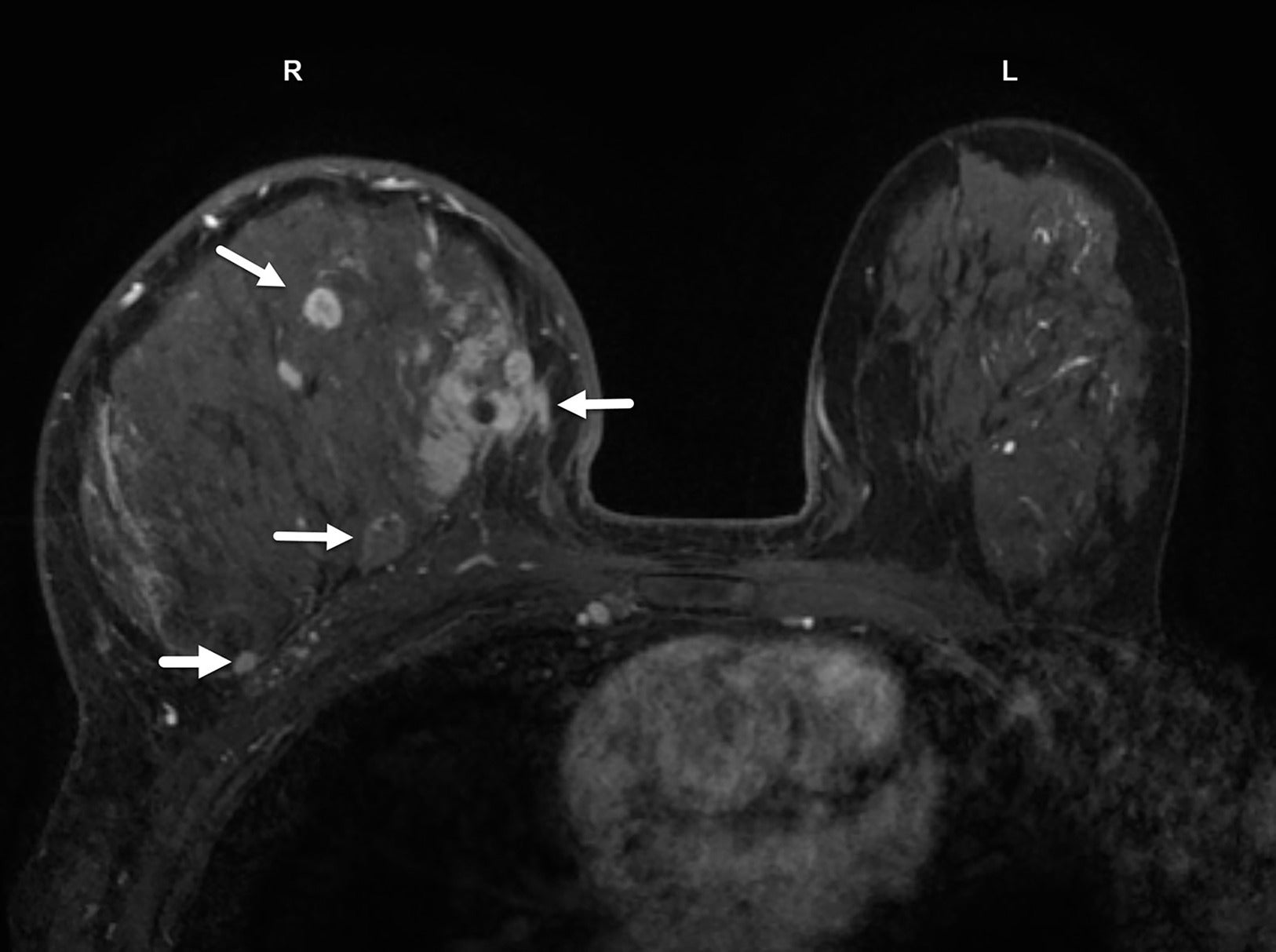
(A, B) Digital, mediolateral oblique mammogram in a senior patient with inflammatory breast cancer shows dense breast with no discrete mass. BI-RADS Category 0: needs additional imaging evaluation. Sagittal MR image of the same patient demonstrates multiple enhancing masses (solid white arrows) with irregular margins in multicentric distribution. Enhancing tumoral masses also extend into the anterior thickened skin (small yellow arrows) and infiltrate the chest wall (dotted blue arrows). BI-RADS Category 5: highly suggestive of malignancy.
The multicentric distribution commonly associated with IBC, especially in dense enlarged and inflamed breasts ( Figure 9 ), may best be appreciated on MRI. Edema of the subdermal breast, pre-pectoral region, and chest wall is more commonly seen in IBC than in other breast cancers and is most evident on T2 images ( Figure 10 ).19 The presence of pre-pectoral edema has been suggested as a prognostic factor in breast cancer.25, 26
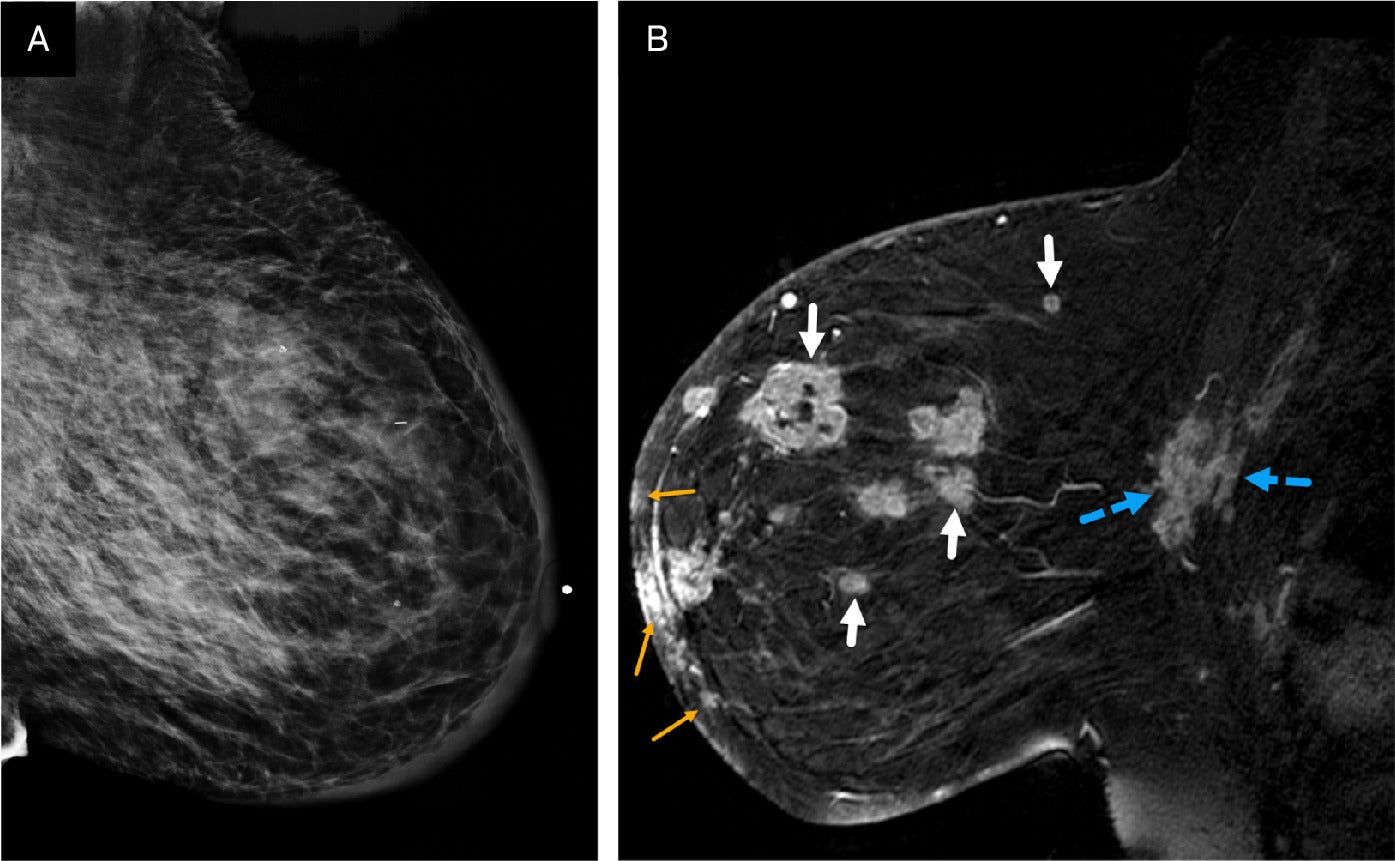
(A, B) Digital left craniocaudal mammogram demonstrates a mass in the lateral breast with irregular margins (solid white arrows) and mild medial skin thickening. BI-RADS Category 4C: high suspicion for malignancy. Axial postcontrast MRI of the same patient reveals additional tumoral lesions distributed multicentrically throughout the rest of the breast (small yellow arrows), along with the mass seen on mammogram (solid white arrow). Diffuse skin thickening is also observed of the left breast. BI-RADS Category 5: highly suggestive of malignancy. The multicentric disease is more conspicuous on the MRI than on the mammogram.
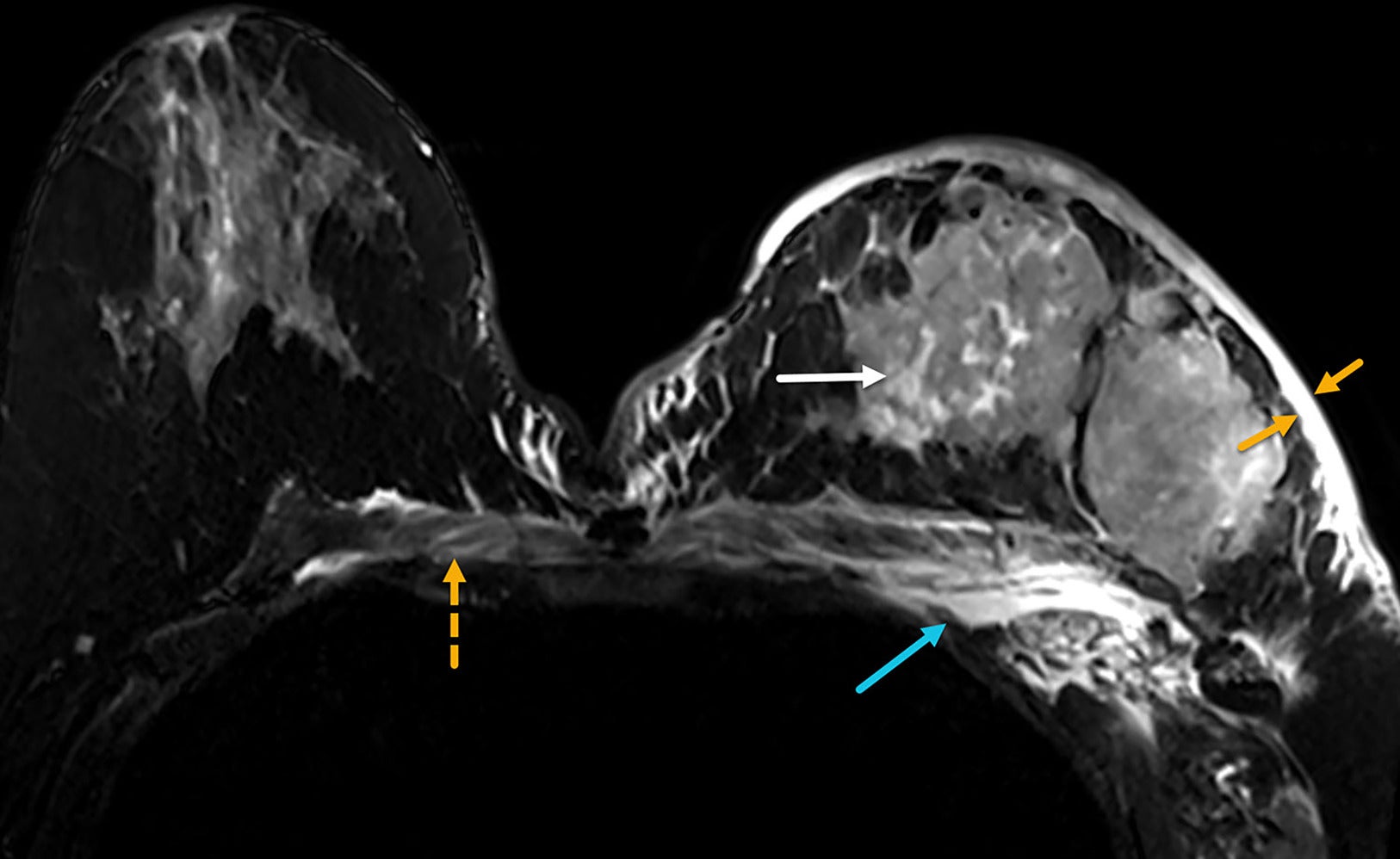
Noncontrast axial T2 MRI of both breasts in a patient with inflammatory breast cancer who presented with a 1-month history of rapid left breast swelling and redness that did not improve with antibiotic therapy. MRI demonstrates edema (bright T2 signals) in the thickened skin (yellow arrows), in the tumoral masses (white arrow), and in the chest wall and subpectoral region (blue arrow). Edema also crosses the midline into the contralateral chest wall (dotted yellow arrow). BI-RADS Category 5: highly suggestive of malignancy.
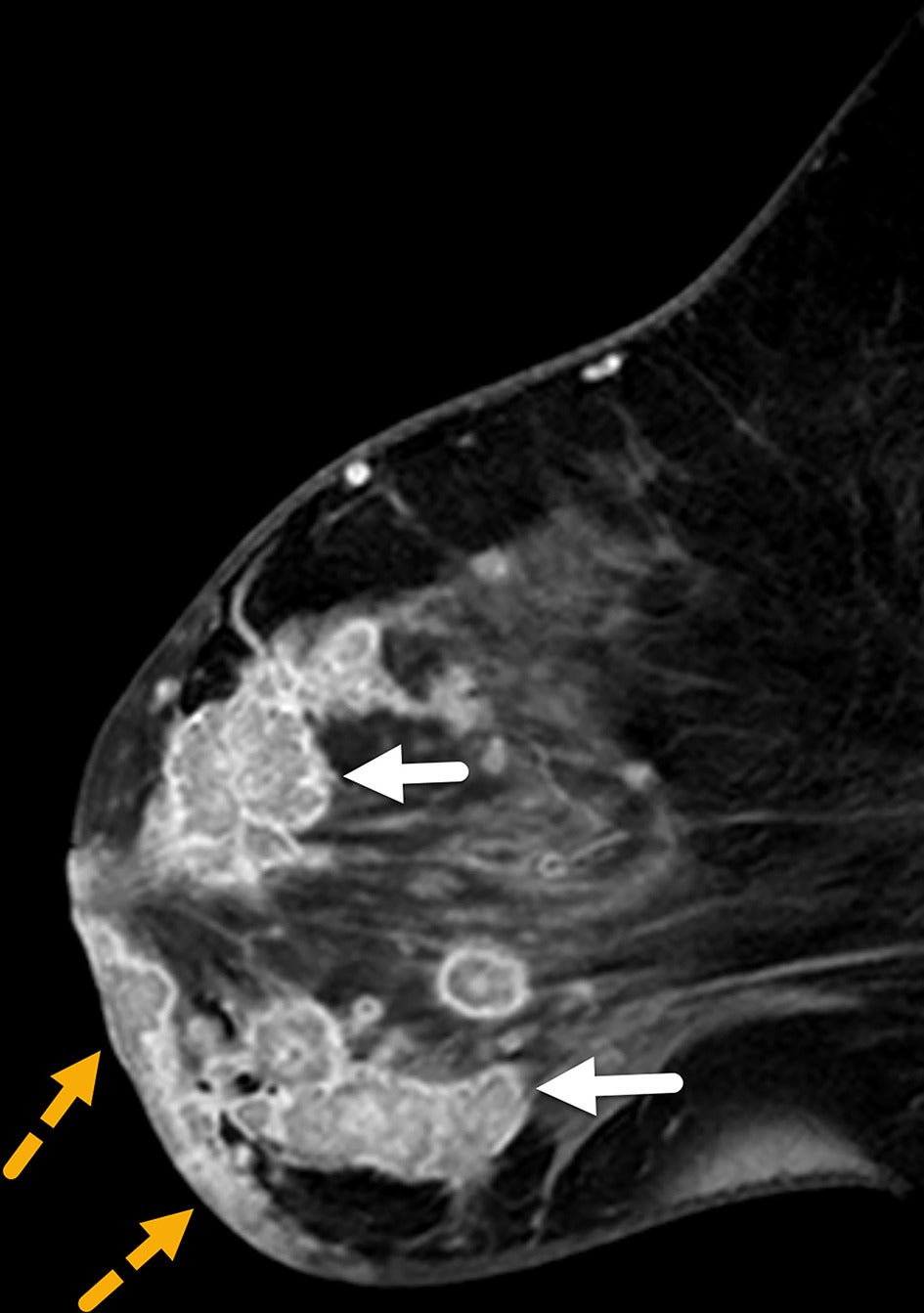
Diffuse skin thickening, observed in 90-100% of patients with IBC, may be present with or without skin enhancement or focal-enhancing skin lesions ( Figure 11 ).19 Skin thickening typically involves the entire breast and may extend across the midline or into the contralateral breast. Focal skin thickening adjacent to the BPL is more often associated with locally advanced or neglected carcinoma than with IBC.27 Enhancing skin foci detected on MRI may represent tumor emboli or dermal lymphatic invasion, pathological hallmarks of IBC.
Sagittal postcontrast MRI of the left breast in a patient with inflammatory breast cancer who initially presented with mastitis-like symptoms not resolved with antibiotics. MRI demonstrates global skin thickening with multiple skin lesions in the dermis of the inferior breast (dotted yellow arrows). Conglomerate of suspicious breast masses are seen in all quadrants (solid white arrows). BI-RADS Category 5: highly suggestive of malignancy.
Nodal Staging
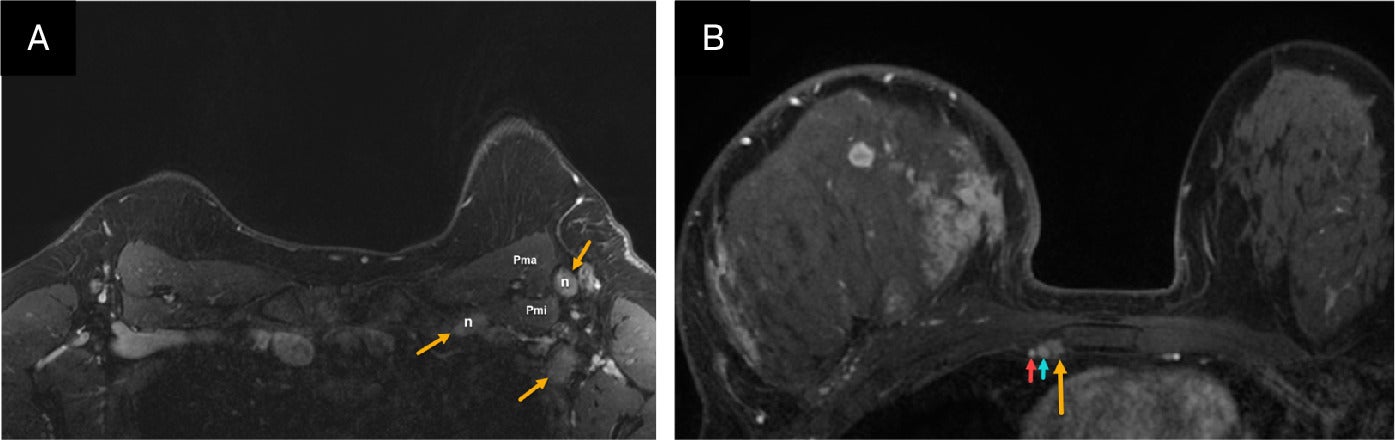
Bilateral nodal basins are visible on MRI despite artifact resulting from cardiac motion and respiration. The axillary level I and II nodal regions, as well as the internal mammary nodal basins, are often well visualized ( Figure 12 ), while the supraclavicular and medial infraclavicular or axillary level III regions are better evaluated with nodal ultrasound.
(A) Axial postcontrast MR image in a patient with inflammatory breast cancer (IBC) shows suspicious left axillary level I and III lymph nodes (solid yellow arrows). Biopsy confirmed metastatic adenopathy. Pma, pectoralis major muscle; Pmi, pectoralis minor muscle. (B) Axial postcontrast MRI in another patient with IBC shows suspicious right internal mammary node (yellow arrow) next to the internal thoracic vein (blue arrow). Biopsy of the internal mammary Internal thoracic artery (red arrow).
18F FDG PET/CT
PET/CT is recommended for patients with IBC at initial presentation, particularly when standard staging studies are inconclusive, and for identifying extra-axillary lymph node metastases ( Figure 13 ) and occult distant disease.28, 29 Since at least one-third of these patients present with distant metastases, the modality is also valuable for guiding treatment selection and determining prognosis.30 PET/CT has demonstrated a sensitivity of 96-100% for PBL in IBC.31, 32 However, false-positive findings are possible in such cases as mastitis, which may show FDG avidity similar to that of IBC.32
Two axial images of the18-F FDG-PET/CT exam in a patient with inflammatory breast cancer reveal right axillary level I, II, and III hypermetabolic nodes (yellow arrow) and contralateral superior mediastinal nodes (green arrow). The patient has multiple hypermetabolic right breast tumoral masses (white arrow) within the enlarged inflamed right breast.
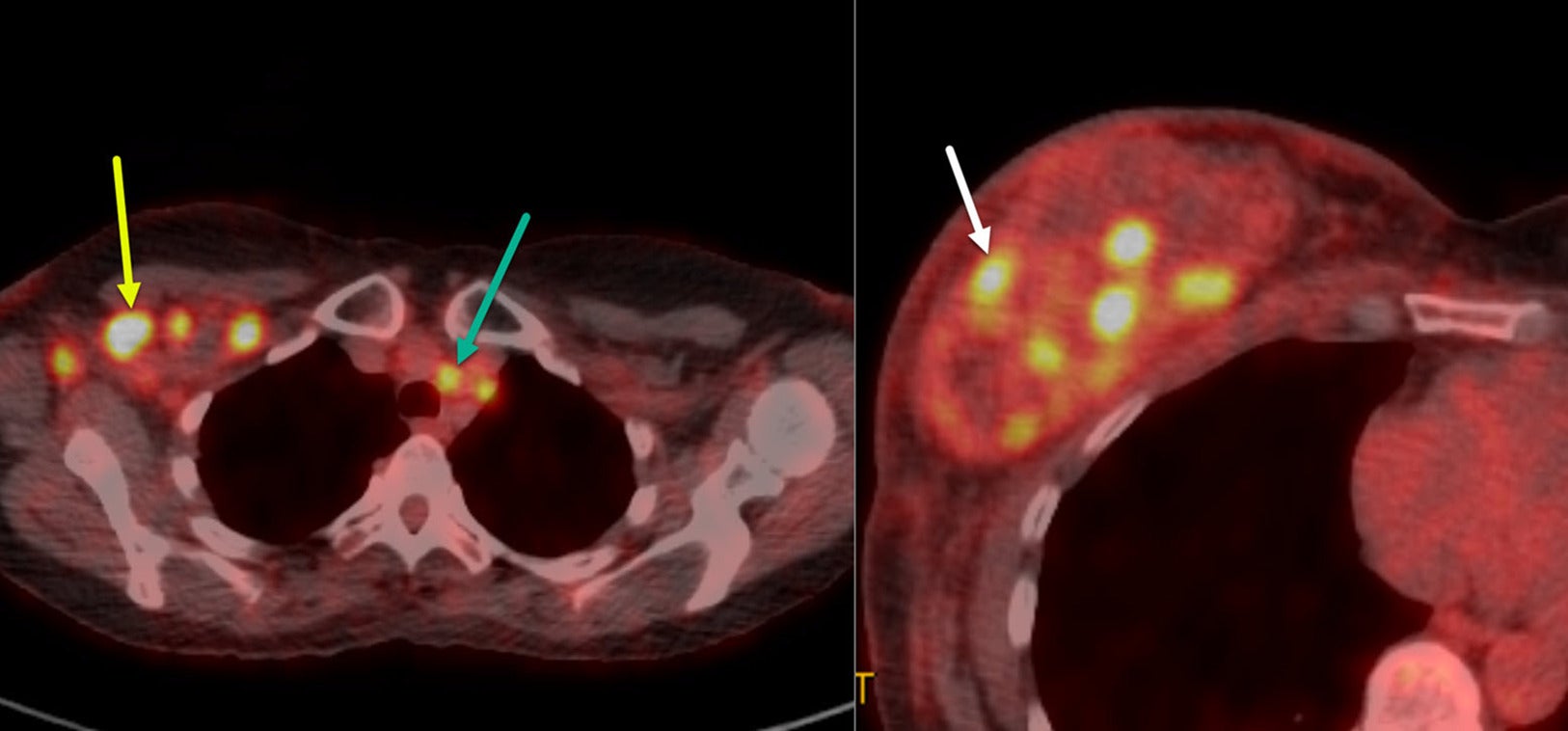
PET/CT is particularly valuable for evaluating regional lymph node metastases in patients with IBC. Studies by Alberini, Groheux, and Caracki et al have highlighted its utility in identifying nodal disease in the axilla, as well as in the subpectoral, internal mammary, and supraclavicular lymph nodes.31 - 34 In addition, PET/CT may identify metastatic disease not evident on clinical examination and/or other imaging modalities.
In addition to regional lymph node assessment, PET/CT is useful in identifying distant nodal disease (eg, mediastinal or contralateral axillary adenopathy) and distant metastases. Studies by Groheux et al and Carkaci et al reported that distant metastases were detected by the modality in 46% and 49% of patients with IBC, respectively.22, 23 It was also superior to CT for distant lymph node, bone, and liver metastases, and it outperformed bone scans in identifying metastases in these tissues.22 However, chest CT was more sensitive for lung and pleural metastases.22 In certain subsets of patients with IBC, such as those with triple-negative breast cancer, visceral metastases, or young age at diagnosis, up to 30% of cases can present with brain metastasis. Therefore, brain MRI is recommended for these patients.34
Treatment
Systemic Chemotherapy
IBC is highly aggressive, with poor survival rates. Before the introduction of systemic chemotherapy, fewer than 5% of patients treated with surgery and/or radiation therapy alone survived beyond 5 years, with a median survival under 15 months.35, 36 Local recurrence rates were high, at approximately 50%, and many patients were candidates for surgery.37 Over the past 2 decades, the consensus treatment for IBC has evolved to include systemic chemotherapy (with trastuzumab and endocrine therapy when indicated), followed by surgery and radiation therapy. Although IBC has been excluded from most prospective chemotherapy trials, retrospective trials have demonstrated the efficacy of systemic chemotherapy in IBC.
Anthracycline-based chemotherapy, introduced in the 1970s, significantly improved outcomes for IBC, achieving clinical response rates up to 72% and increasing 5- and 10-year survival rates compared with earlier treatments.38 - 44 Combining anthracycline-based chemotherapy with taxanes has further enhanced these responses. Studies at the MD Anderson Cancer Center that added paclitaxel to standard regimens increased pathological complete response (pCR) rates.39 - 43 Achieving pCR, particularly in the axillary lymph nodes, remains the most significant prognostic factor for long-term survival.44
Approximately 17-30% of IBC cases are triple-negative; that is, the tumor lacks estrogen and progesterone receptors and HER2 overexpression.45, 46 For these patients, adding pembrolizumab to neoadjuvant anthracycline- and taxane-based systemic chemotherapy has demonstrated improved pCR rates.47 This combination is now widely used for triple-negative IBC.
HER2-positive IBC accounts for 36-60% of cases and benefits significantly from trastuzumab-based regimens.48, 49 The NOAH trial demonstrated that adding trastuzumab to standard chemotherapy significantly increased the pCR rate in patients with HER2-positive IBC.50 Other studies corroborate these findings, suggesting that trastuzumab is essential to treatment in these cases.51, 52
No standard IBC-specific treatments currently exist for patients with advanced or metastatic disease. This highlights the importance of clinical trials, including those focusing on exploratory or novel targeted therapies for patients with advanced or metastatic IBC.
Surgery
Total mastectomy with axillary lymph node dissection (modified radical mastectomy) is recommended for patients with IBC. The optimal timing for surgery is 3-6 weeks after the completion of neoadjuvant systemic therapy. The primary goal is to excise all macroscopic diseases and obtain pathologically clear margins. Excising grossly abnormal skin is also recommended; in patients where primary closure is not possible, advanced wound coverage techniques, such as skin grafting or myocutaneous flap closure with assistance from plastic surgery, may be indicated. Because breast skin excision is necessary for patients with IBC, immediate reconstruction is contraindicated. An MD Anderson Cancer Center study evaluating long-term outcomes in patients who completed trimodal therapy (neoadjuvant systemic therapy, modified radical mastectomy, and radiation) showed durable survival and a local recurrence rate of 6.9%, which is comparable to that of non-IBC patients.52 Metastatic spread to the regional nodes is noted at presentation in most patients; axillary dissection is recommended in these cases. De-escalation measures such as breast conservation and limited axillary surgeries, which have not been well studied in patients with IBC, may be associated with increased rates of local recurrence.53 As such, total mastectomy with axillary dissection remains the recommended approach to these cases.
Radiation Therapy
All patients should be offered radiation therapy regardless of treatment response. Radiation targets the chest wall and undissected draining lymphatics, with an extra dose (boost) to the chest wall and any undissected clinical stage N3 disease (infraclavicular, supraclavicular, or internal mammary lymph nodes). Dose fractionation details can be found online.54 While historical locoregional control rates are low, at approximately 80% over 5 years, recent data show significant improvement with a risk-stratified approach.54 It is important to note that patients with IBC were excluded in recently presented clinical trials of de-escalation treatment, including hypofractionation and observation after pCR. Therefore, these approaches should be avoided in these patients.
Conclusion
IBC is a rare and aggressive form of advanced breast cancer characterized by rapid progression and distinct skin findings. Accurate, timely diagnosis is essential as skin findings overlap with benign pathologies such as infection and mastitis. Standard-of-care confirmatory imaging tests consist of mammography, breast and nodal ultrasound, and MRI. PET/CT is instrumental in detecting distant metastases and disease staging. Standard-of-care treatment involves a multimodal approach with chemotherapy, mastectomy with axillary lymph node dissection, and radiation therapy. Despite advances in imaging technology and emerging chemotherapies, IBC survival rates remain poor, underscoring the importance of continued research and encouraging participation in clinical trials.
References
Citation
Le-Petross HT, FRCPC, FSBI, Salem S, Kapoor M, Sun S, Team, AIBC, Woodward WA.Comprehensive Review of Clinical Presentation, Multimodality Imaging, and Therapeutic Strategies for Inflammatory Breast Cancer. Appl Radiol. 2025; (3):
doi:10.37549/AR-D-25-0082
June 1, 2025