Chronic Mesenteric Ischemia: Mesenteric Artery Duplex Sonography and the Utility of Postprandial Imaging
Images
Chronic mesenteric ischemia (CMI), also referred to as abdominal angina, is characterized by gradually worsening perfusion of the bowel as a consequence of narrowing of the celiac artery (CA), superior mesenteric artery (SMA), and/or inferior mesenteric artery (IMA).1 The majority of the small and large bowel is supplied by branches of the SMA; however, owing to the significant collateral blood supply of the mesentery, typically more than one mesenteric artery must be compromised to manifest symptoms of CMI. Most often the result of atherosclerosis, CMI also may sometimes be caused by fibromuscular dysplasia and vasculitis.1 Risk factors include increased age, female gender, and comorbidities that predispose to atherosclerosis, such as smoking and diabetes. Patients often present with postprandial pain, weight loss, and food aversion.2
The American College of Radiology (ACR) Appropriateness Criteria® states that abdominal duplex sonography “may be appropriate” as initial imaging in symptomatic patients with suspected CMI.3 However, the potential drawbacks of ultrasonography of the mesenteric vasculature, such as technical difficulty in visualization due to bowel gas, are considered in the ACR’s recommendations. Computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) of the abdomen and pelvis are considered “usually appropriate” per the ACR Appropriateness Criteria®, although CTA has proven to be most accurate, with the highest inter-reader agreement relative to MRA and ultrasound.3
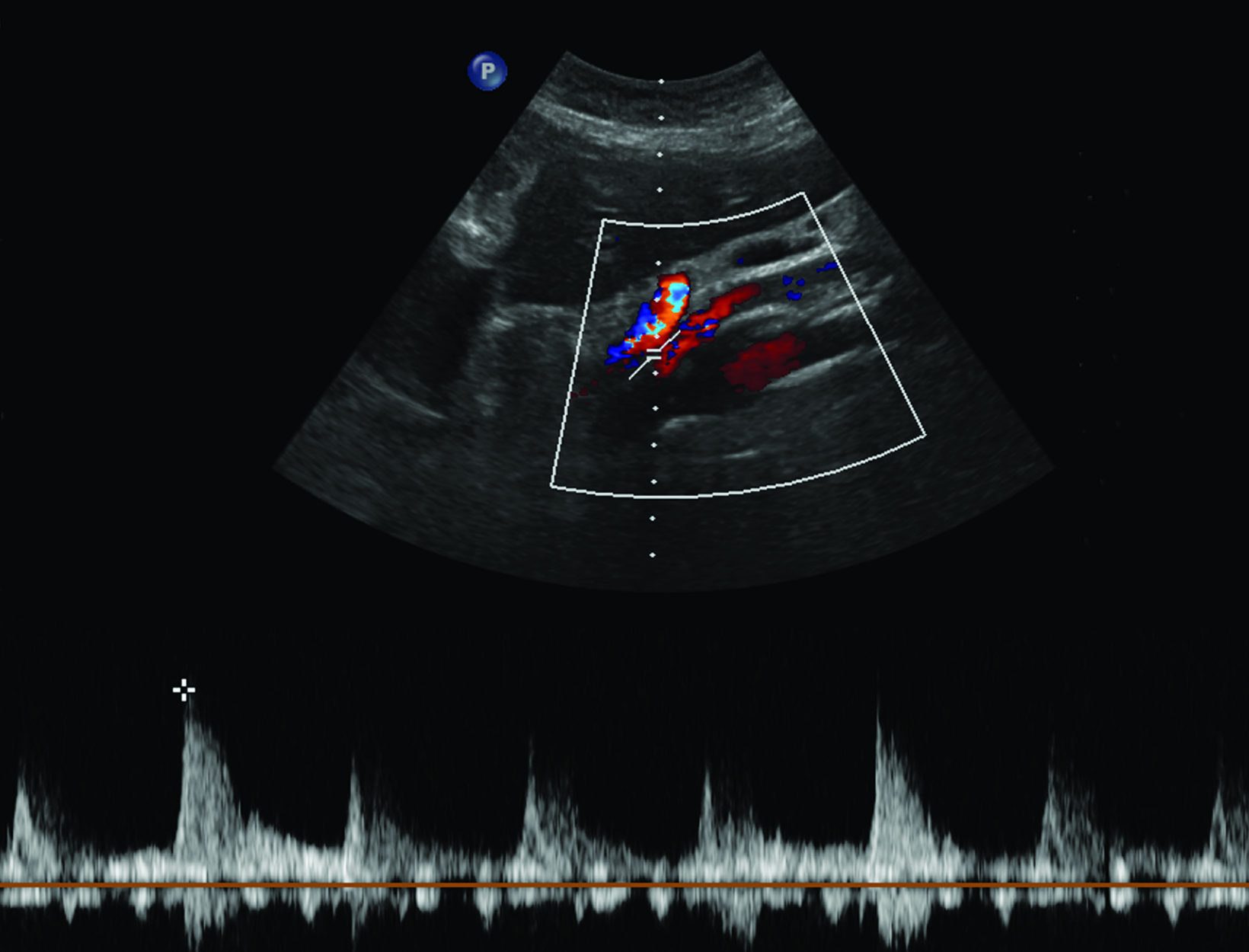
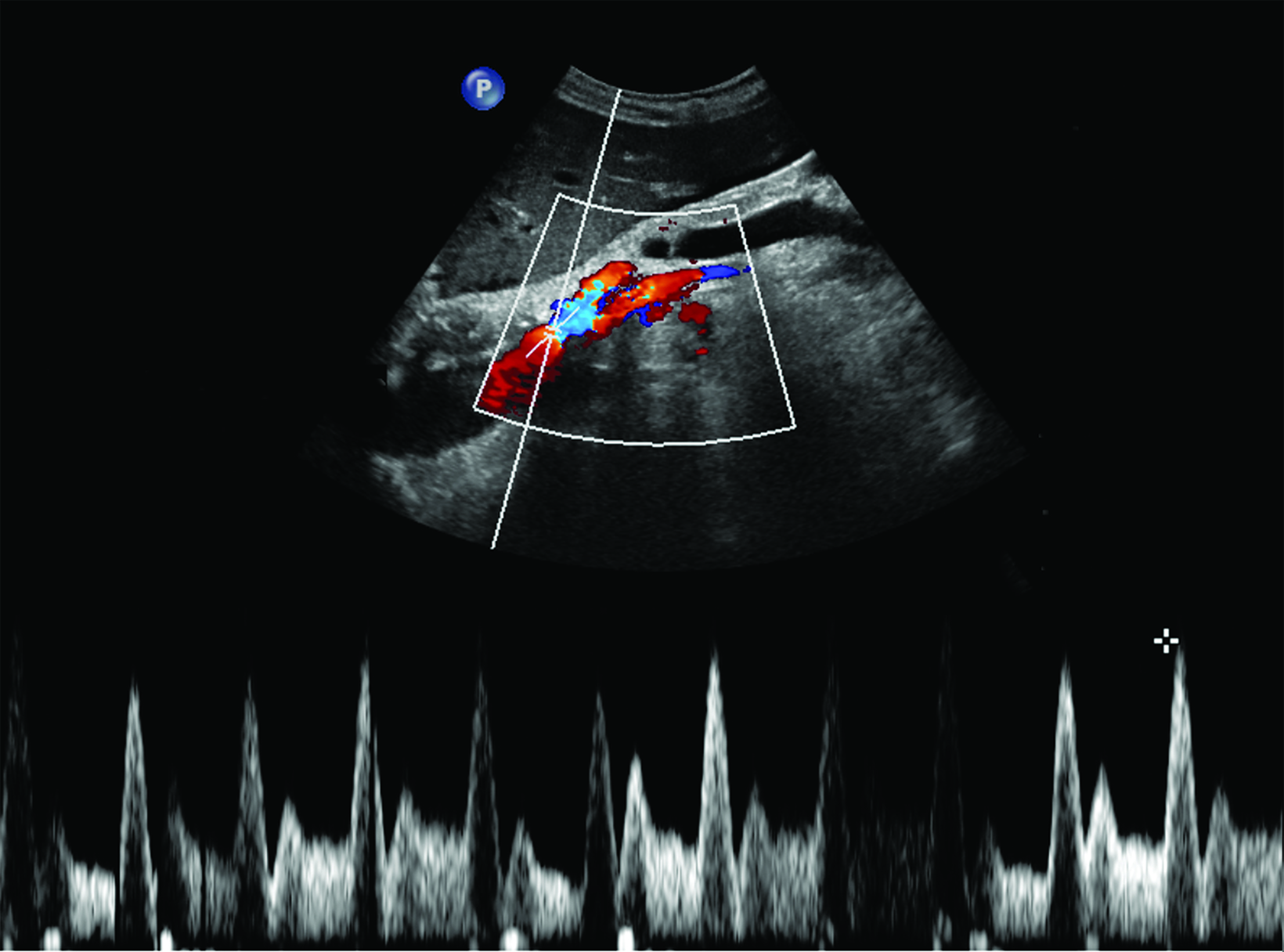
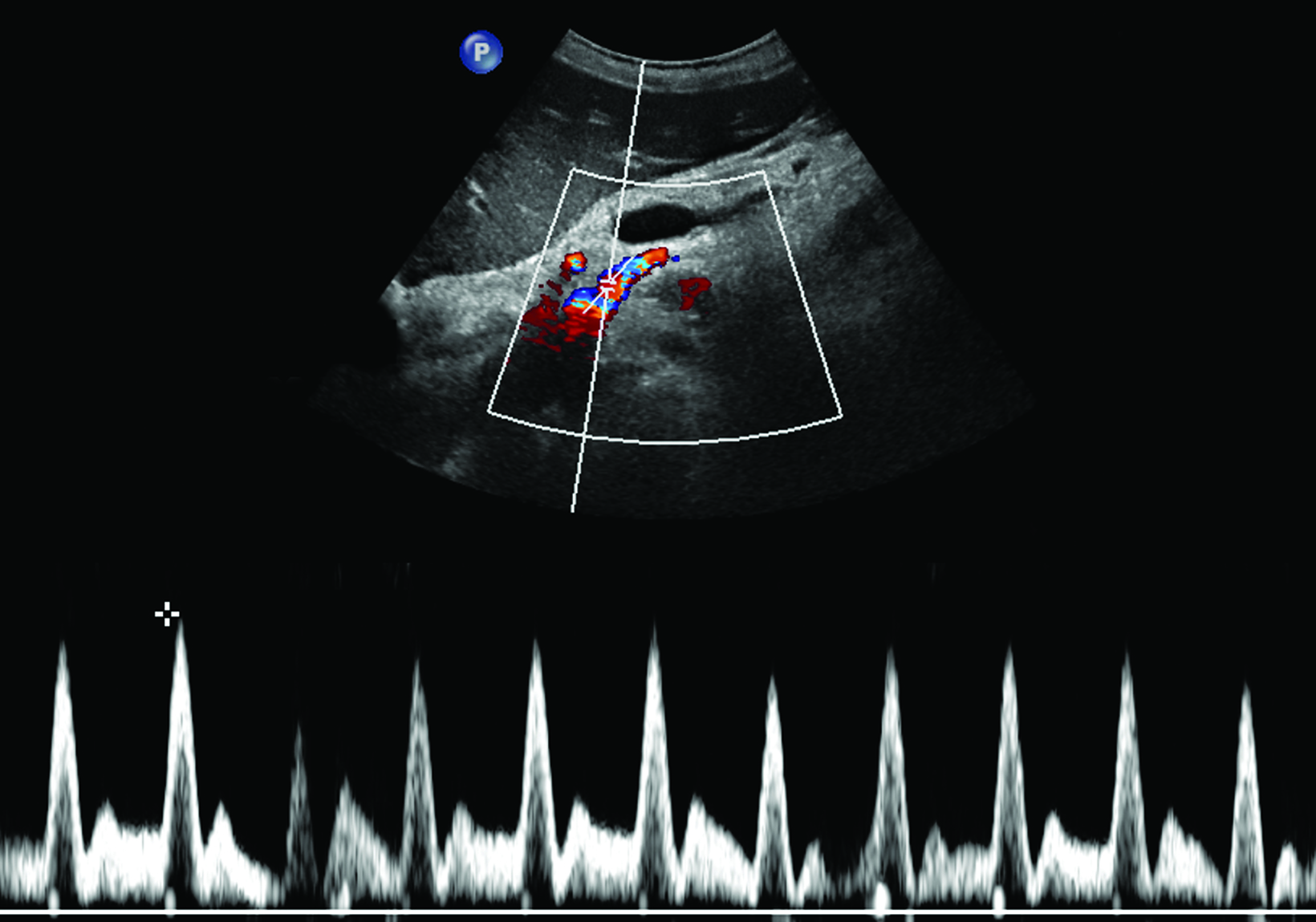
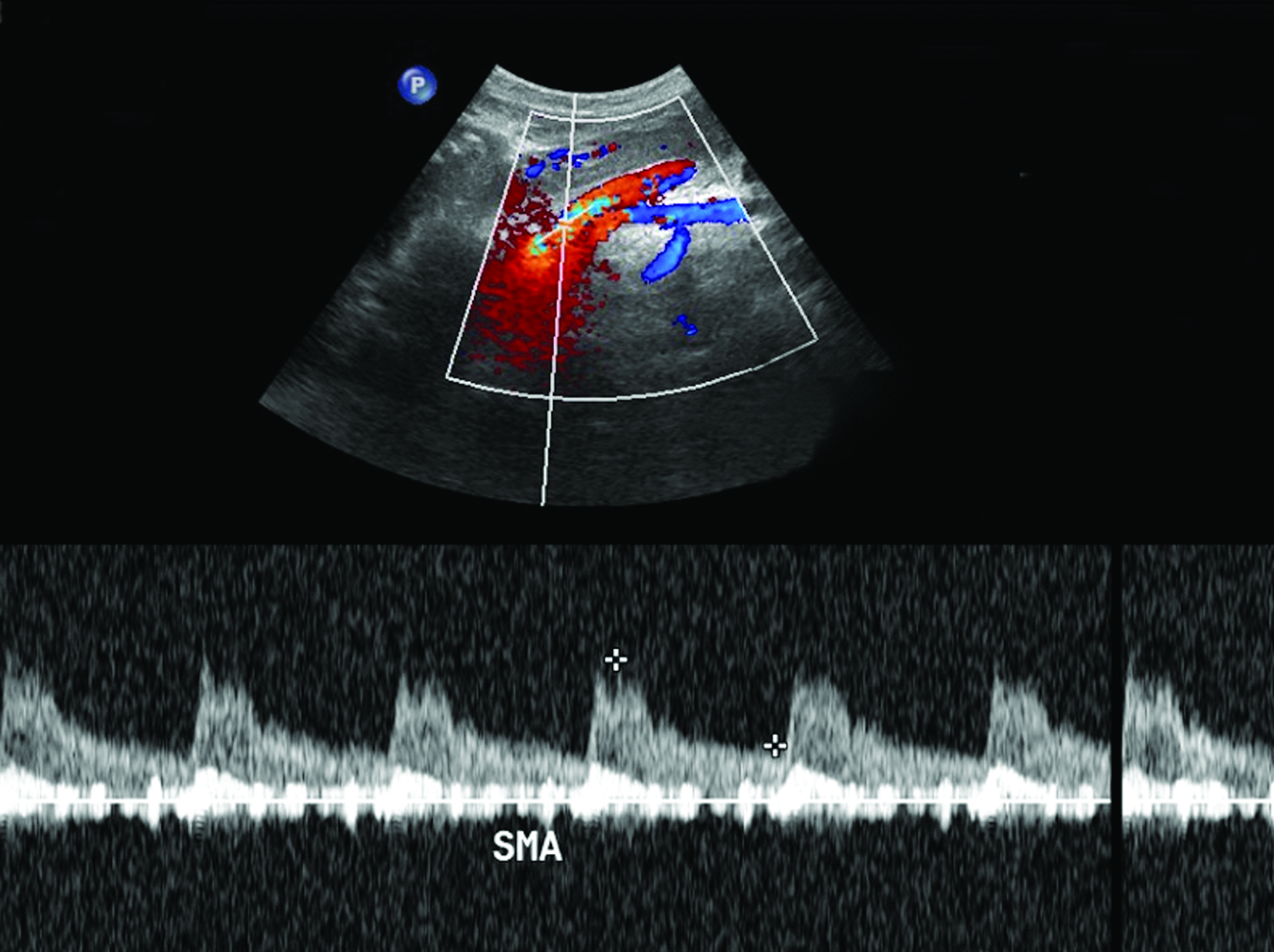
Ultrasonography is a cost-effective and noninvasive way to evaluate for CMI in symptomatic patients. The examination consists of grayscale and color Doppler imaging of the aorta, CA, SMA, and IMA, in addition to spectral Doppler evaluation with measurement of the peak systolic velocity (PSV) at the proximal portion of each artery (Figure 1). End-diastolic velocity (EDV) and PSV ratios relative to the aorta may also be obtained based on institutional protocol. Ultrasound may provide additional information regarding functional flow limitations that will not be evident on nondynamic CTA or MRA imaging.
Ultrasound Evaluation of Chronic Mesenteric Ischemia in the Fasting State
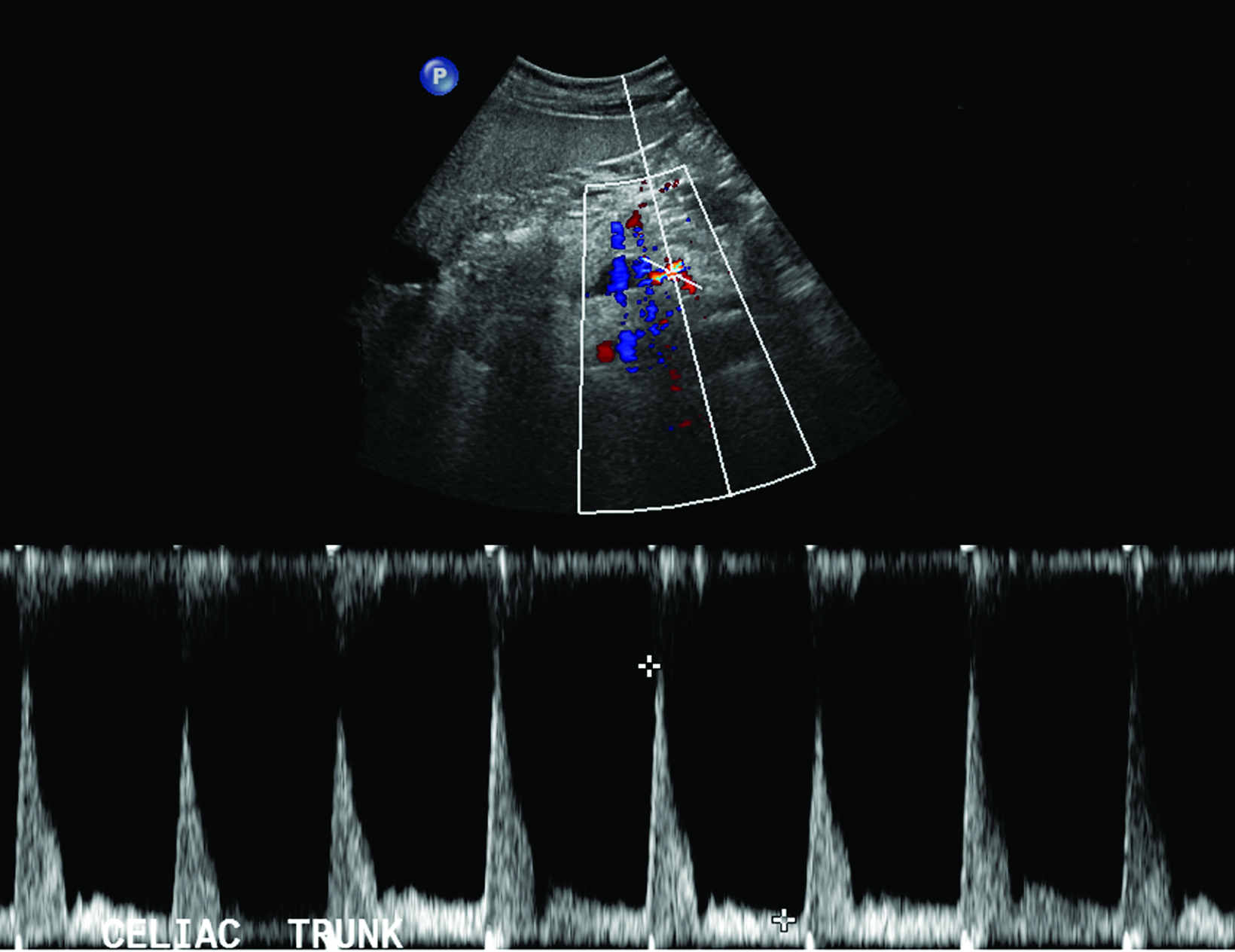
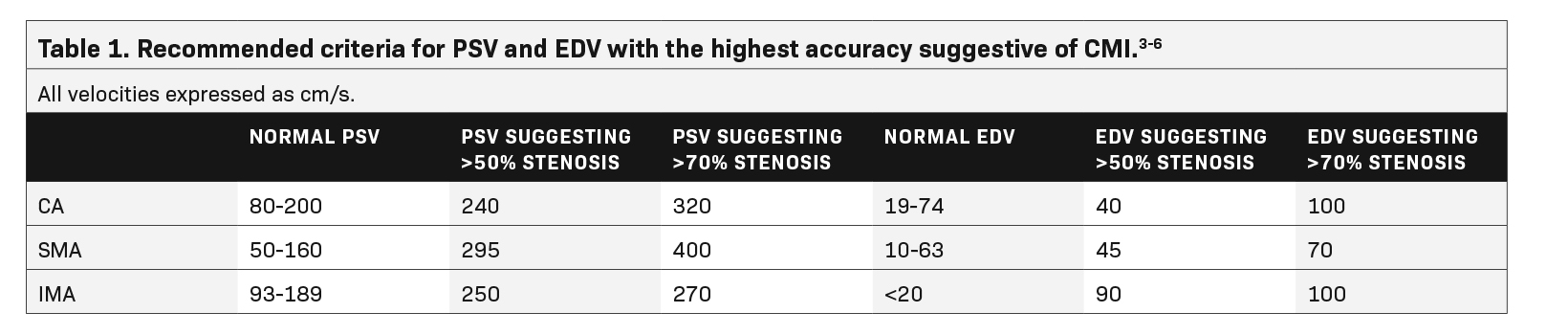
Sonographic examination is best performed in the fasting state to reduce artifact and interference from bowel gas.2 Although no universally accepted criteria or standards exist for diagnosing CMI based on fasting PSV, multiple studies recommend a cutoff ranging from 200-320 cm/s in the CA and 275-400 cm/s in the SMA to be suggestive of at least 70% stenosis in these vessels (Table 1).3,4
A 2012 study by AbuRahma et al found PSV values to be more accurate in discerning hemodynamically significant arterial stenosis of greater than 50% and greater than 70% compared to EDV and SMA or CA/aorta PSV ratios.4 Cutoff PSV values of 295 cm/s and 400 cm/s in the SMA correspond to >50% (sensitivity 87%, specificity 89%, accuracy 88%) and >70% stenosis (sensitivity 72%, specificity 93%, accuracy 85%), respectively (Table 1).4 Slightly lower PSV measurements are used as the cutoff in the CA, with PSV of 240 cm/s and 320 cm/s correlating with >50% (sensitivity 87%, specificity 83%, accuracy 86%) and >70% stenosis (sensitivity 80%, specificity 89%, accuracy 85%), respectively (Table 1).4 End-diastolic velocities have been found to have markedly lower sensitivity for hemodynamically significant stenosis, ranging from 58-84%, although some institutions continue to obtain these measurements as part of their imaging protocol.4
Peak systolic velocity ratios of the interrogated vessel and aorta can also be utilized to assess for functional flow limitations secondary to stenosis relative to the aorta. Superior mesenteric artery/aorta PSV ratios of 3.5 and 4.5 in the SMA correspond to >50% (sensitivity 69%, specificity 78%, accuracy 73%) and >70% stenosis (sensitivity 67%, specificity 83%, accuracy 78%), respectively.4 Conversely, CA/aorta PSV ratios of 2.75 and 4.5 in the SMA correspond to >50% (sensitivity 81%, specificity 71%, accuracy 78%) and >70% stenosis (sensitivity 75%, specificity 87%, accuracy 82%), respectively.4 Although renal/aorta PSV ratios are commonly used for assessing renal artery stenosis, they are generally not as accurate as the PSV in detecting ≥50% and ≥70% stenosis of the SMA and ≥50% stenosis of the CA.4
Postprandial Ultrasound in the Assessment of Chronic Mesenteric Ischemia
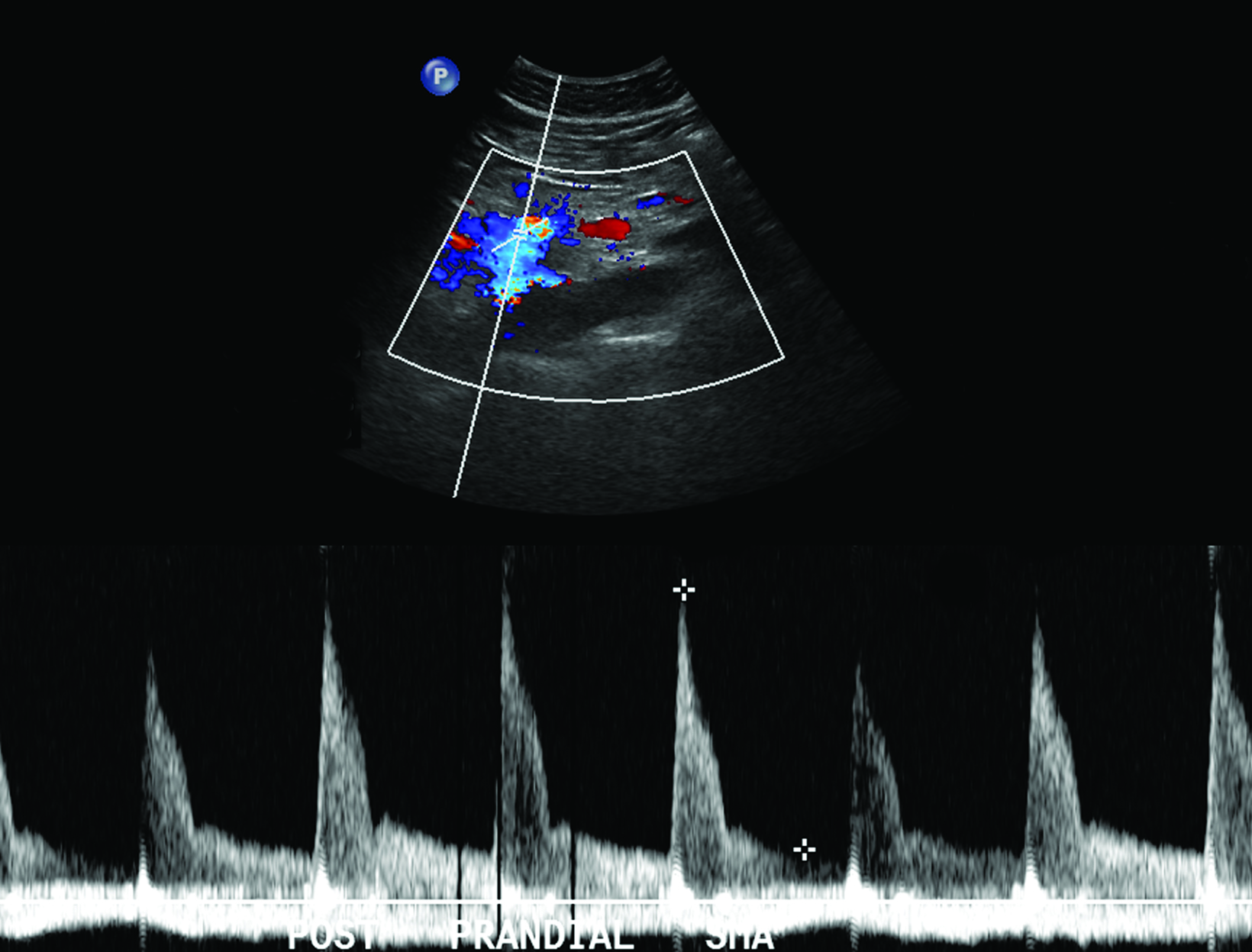
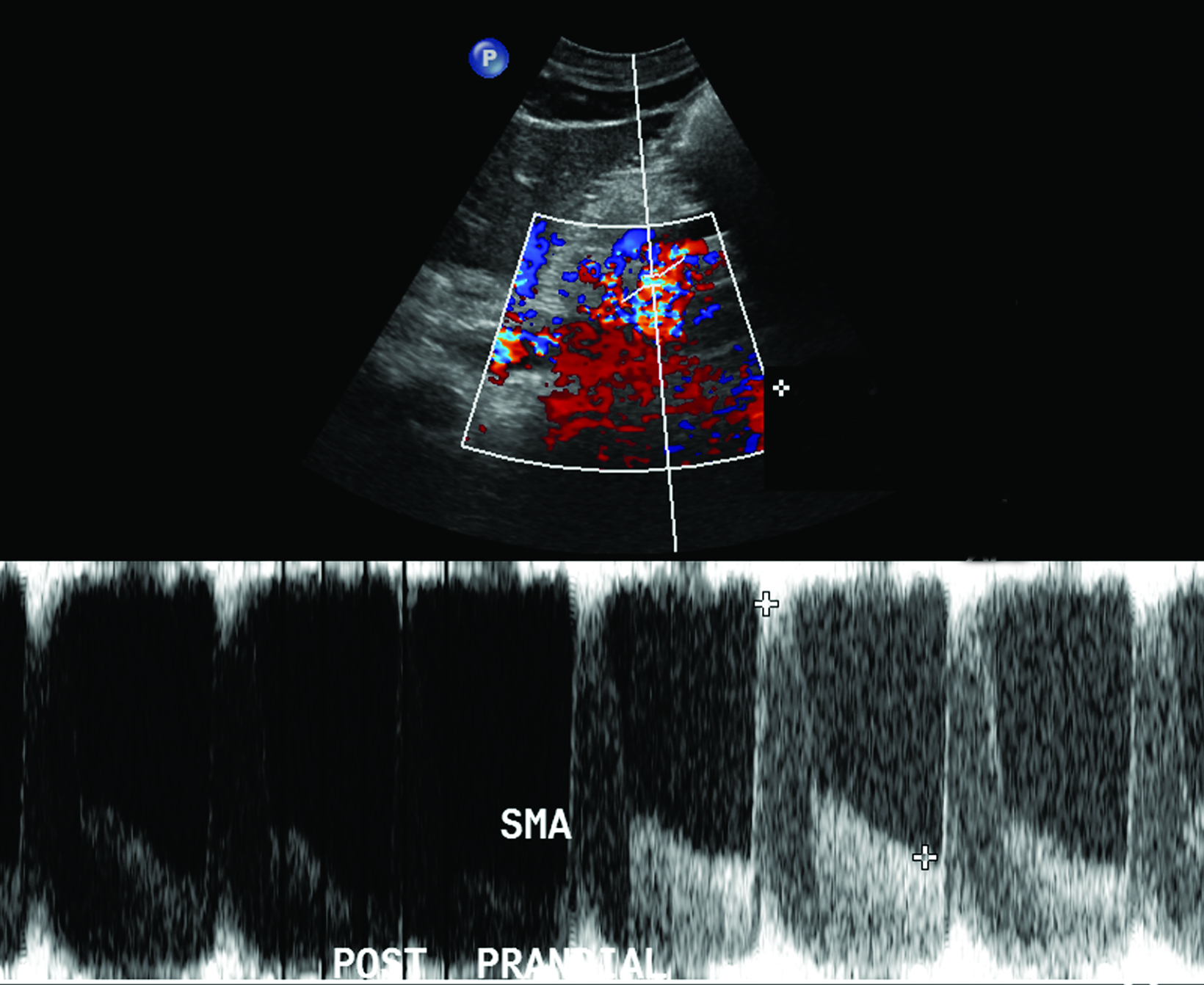
The role of postprandial sonography remains controversial, although several studies have documented its potential utility. Fasting sonogram findings are typically sufficient to suggest a diagnosis of CMI, although postprandial imaging may be performed as a sort of “stress test,” analogous to a cardiac stress test to evaluate for coronary artery disease. Typically, a standard 250-calorie, mixed liquid meal (240 mL) is rapidly ingested by the patient prior to postprandial imaging.6,7
However, imaging performed in the postprandial state often results in fluctuations in PSV measurements, which may lead to lack of reproducibility and variations in interpretation.7 For instance, a 1995 study by Gentile et al found that the combination of fasting and postprandial duplex ultrasound was less sensitive than fasting mesenteric duplex ultrasound alone.8 Furthermore, the literature on postprandial mesenteric arterial sonography is limited and its utility has not been clearly demonstrated.8,9
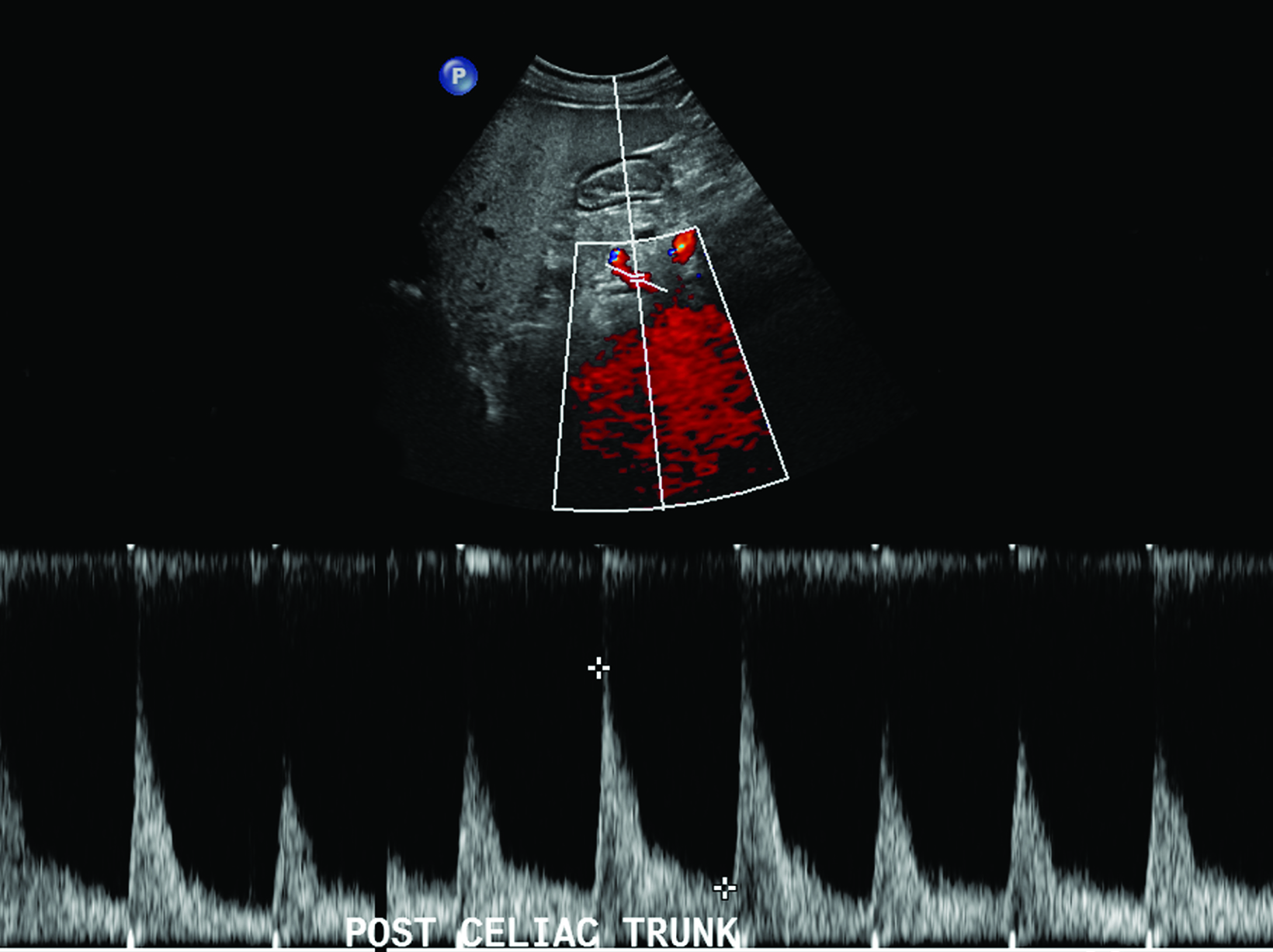
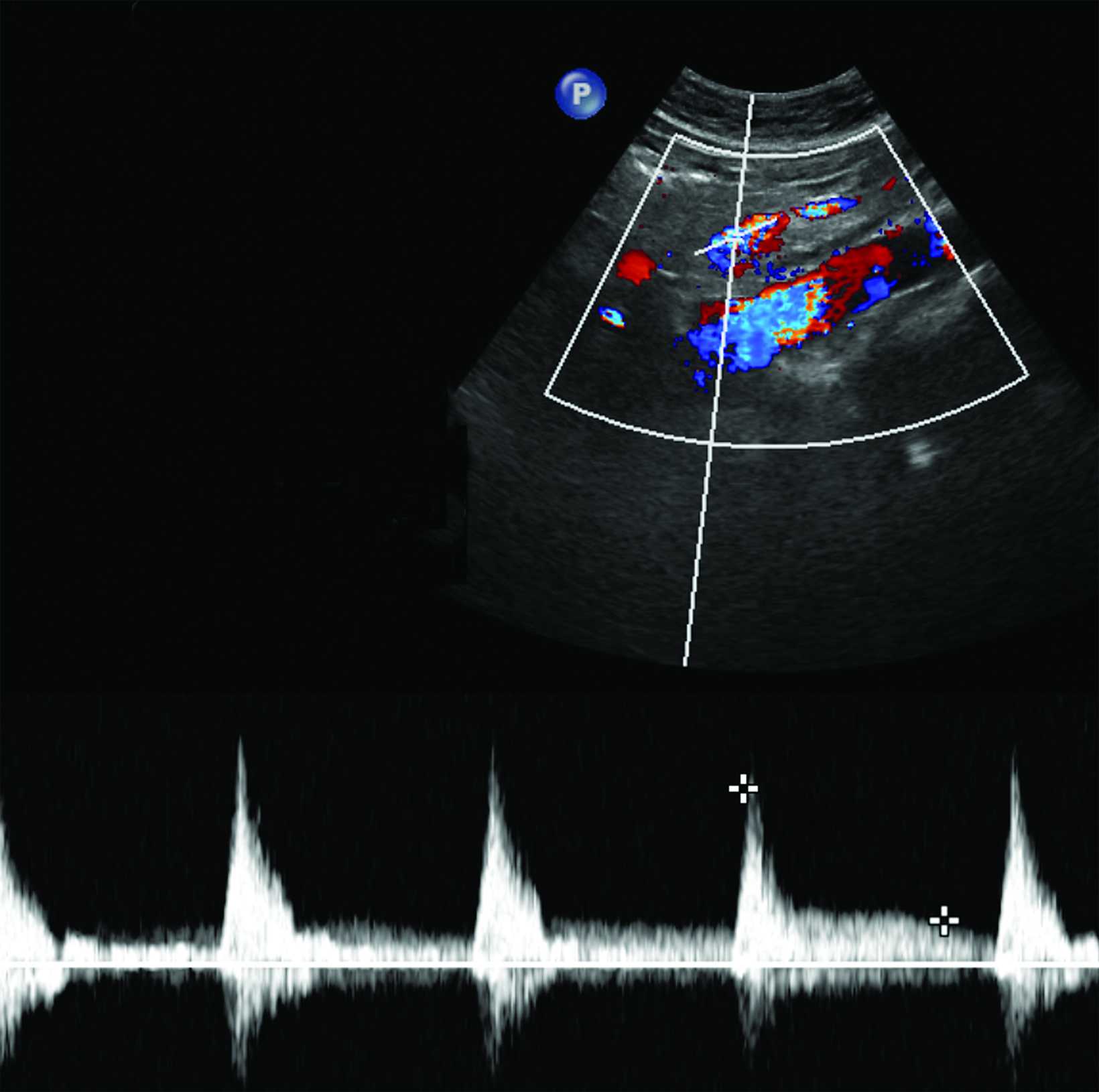
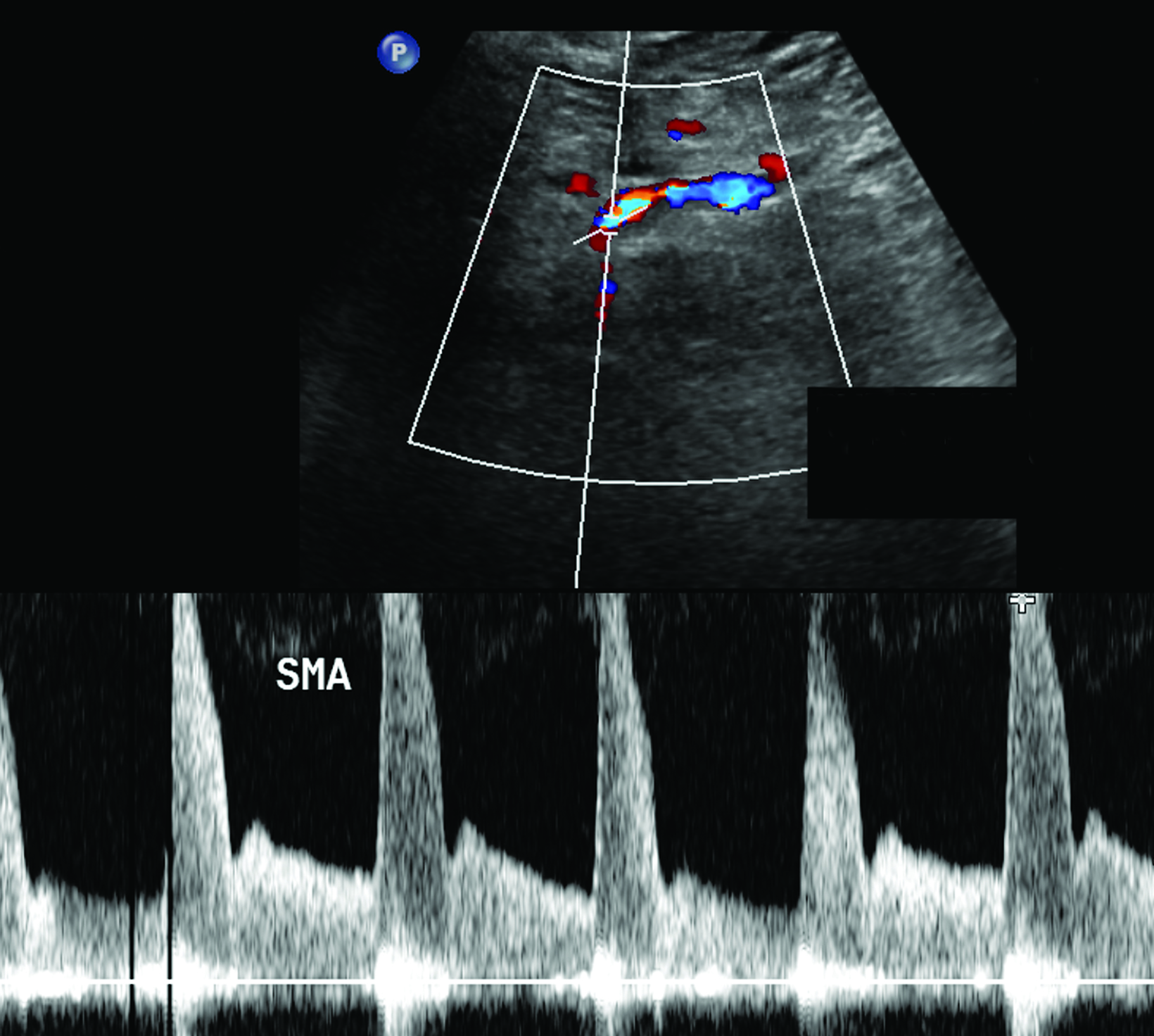
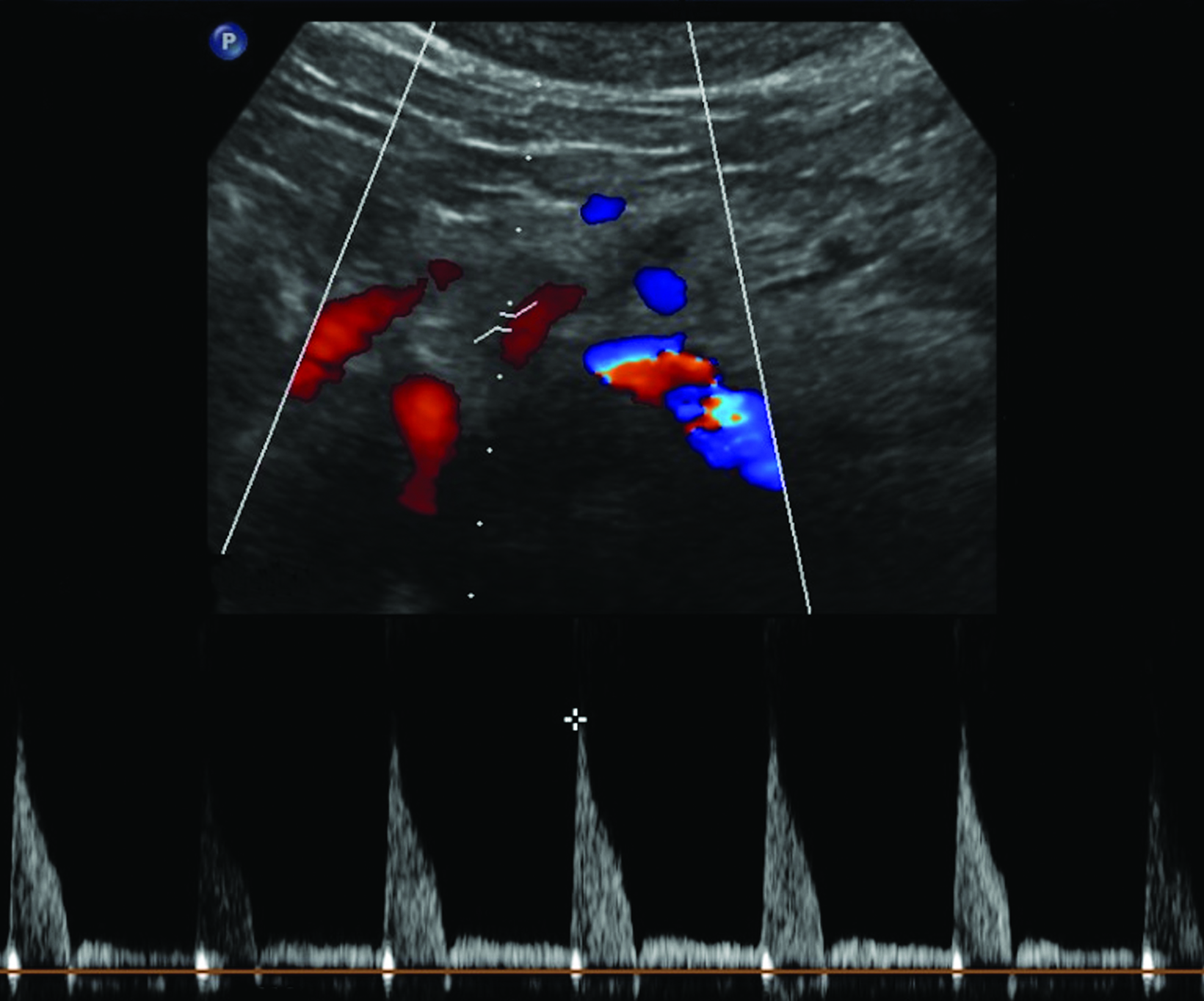
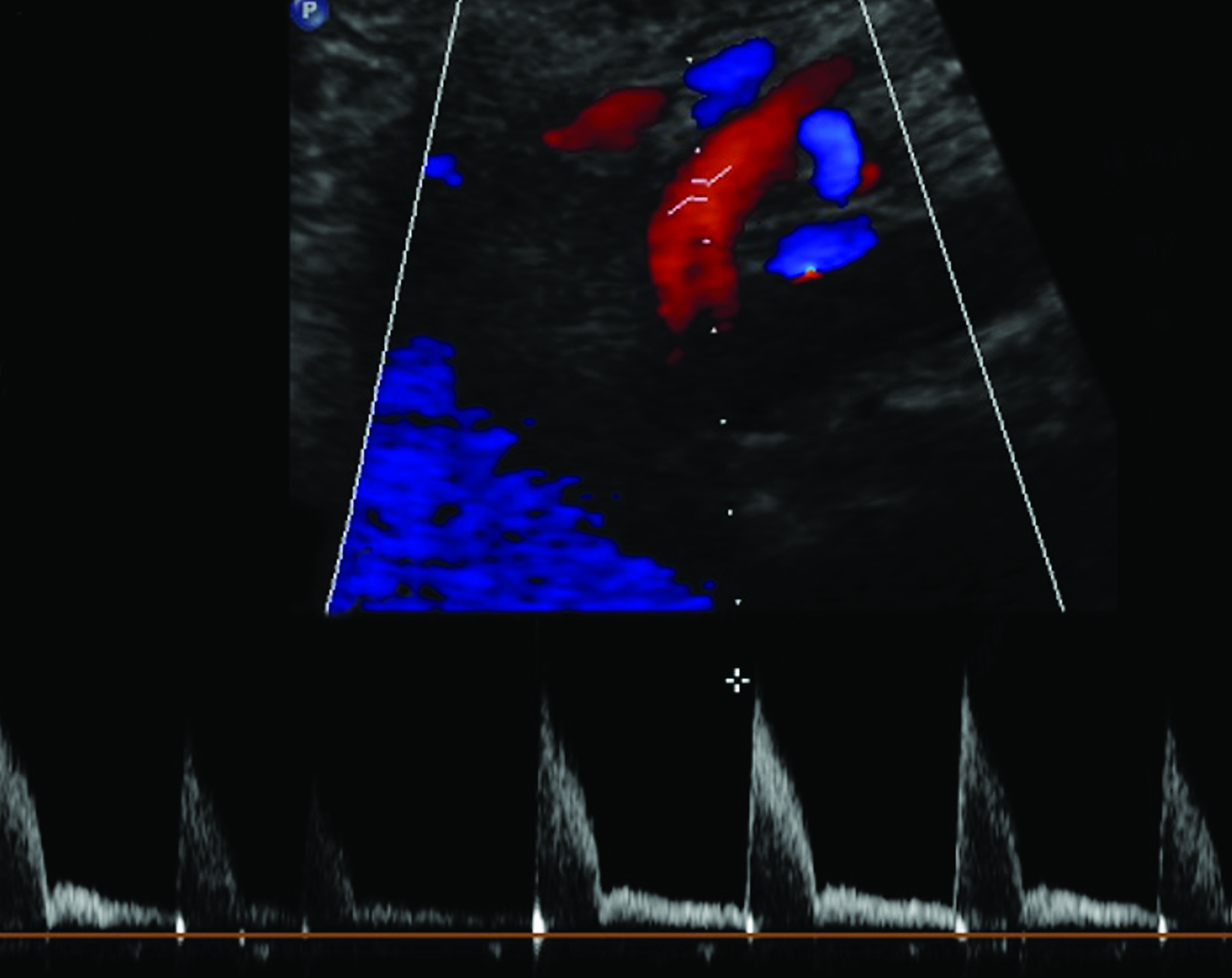
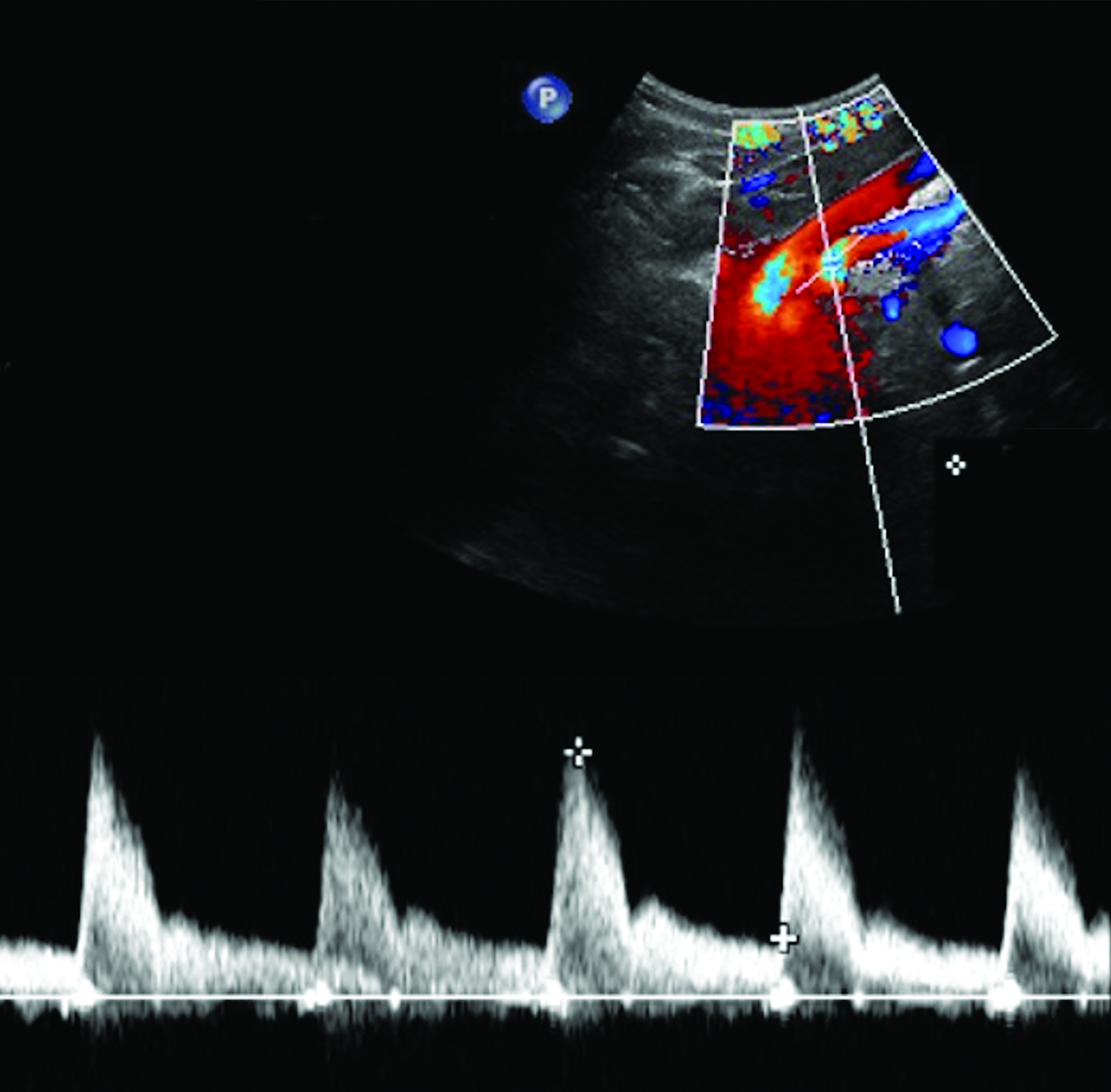
The CA demonstrates a low-resistance pattern with EDV and continuous forward flow during both systole and diastole with blood flow pattern independent of food intake (Figures 2,3).7 The SMA and IMA demonstrate a high-resistance pattern with low diastolic velocities in the fasting state (Figures 4,5). The normal physiological response to a meal is vasodilation of the mesenteric arteries to allow for increased blood flow during digestion, resulting in elevation of PSV and EDV of the mesenteric arteries.7 Some studies have found that both velocities increase after a meal, with at least a doubling of the SMA EDV, while others suggest a modest physiological increase in PSV of 20%-30%.8,10 Overall, increased postprandial velocities are suggestive of patency of the mesenteric arteries. In cases of mesenteric arterial stenosis, the normal postprandial velocity increase would be blunted, resulting in insufficient circulation to the bowel. The failure of PSV to increase at least 20%-30% between fasting and postprandial states is indicative of greater than 70% hemodynamically significant stenosis (Figure 6).8,9
Previous studies have demonstrated that postprandial imaging provides useful information regarding functional flow limitation in symptomatic patients with otherwise normal fasting imaging; the hemodynamic stress of feeding has been proposed as a source of additional information in symptomatic patients.11 A 2013 study by Zachrisson et al found that, in patients with postprandial pain, PSV did not significantly increase after feeding when compared to those without postprandial pain, although mesenteric stenosis was not confirmed by CT or catheter angiography, suggesting that postprandial imaging may provide information about collateral reserve flow capacity.12
In theory, postprandial imaging may provide additional information on functional flow to the bowel after caloric stress, given that the criteria used to diagnose stenosis on postprandial images does not include collateral vasculature. However, the potential exists for a high rate of false positives. Collateral vessels, such those of the pancreaticoduodenal arcade, allow for adequate perfusion of the bowel despite hemodynamically significant stenosis in the primary feeder artery.8,12 Therefore, if bowel perfusion results primarily from compensatory flow through collateral vessels, flow velocity in the primary artery may not increase after the caloric bolus, resulting in a false positive finding.
Reliance on postprandial PSV elevation as a measure of vessel patency may be skewed, as compensatory collateral circulation, such as via the pancreaticoduodenal arcade, may allow for adequate perfusion and an increase in postprandial PSV despite critical proximal stenosis.12 Additionally, including postprandial sonography in the protocol prolongs examination and risks increasing nondiagnostic images due to increased bowel gas. Despite its demonstrated potential utility, postprandial imaging of the mesenteric arteries is not routinely recommended given the aforementioned potential confounding factors and limited additional useful information, especially considering the availability of more advanced imaging techniques.9
Limitations of Mesenteric Artery Ultrasound
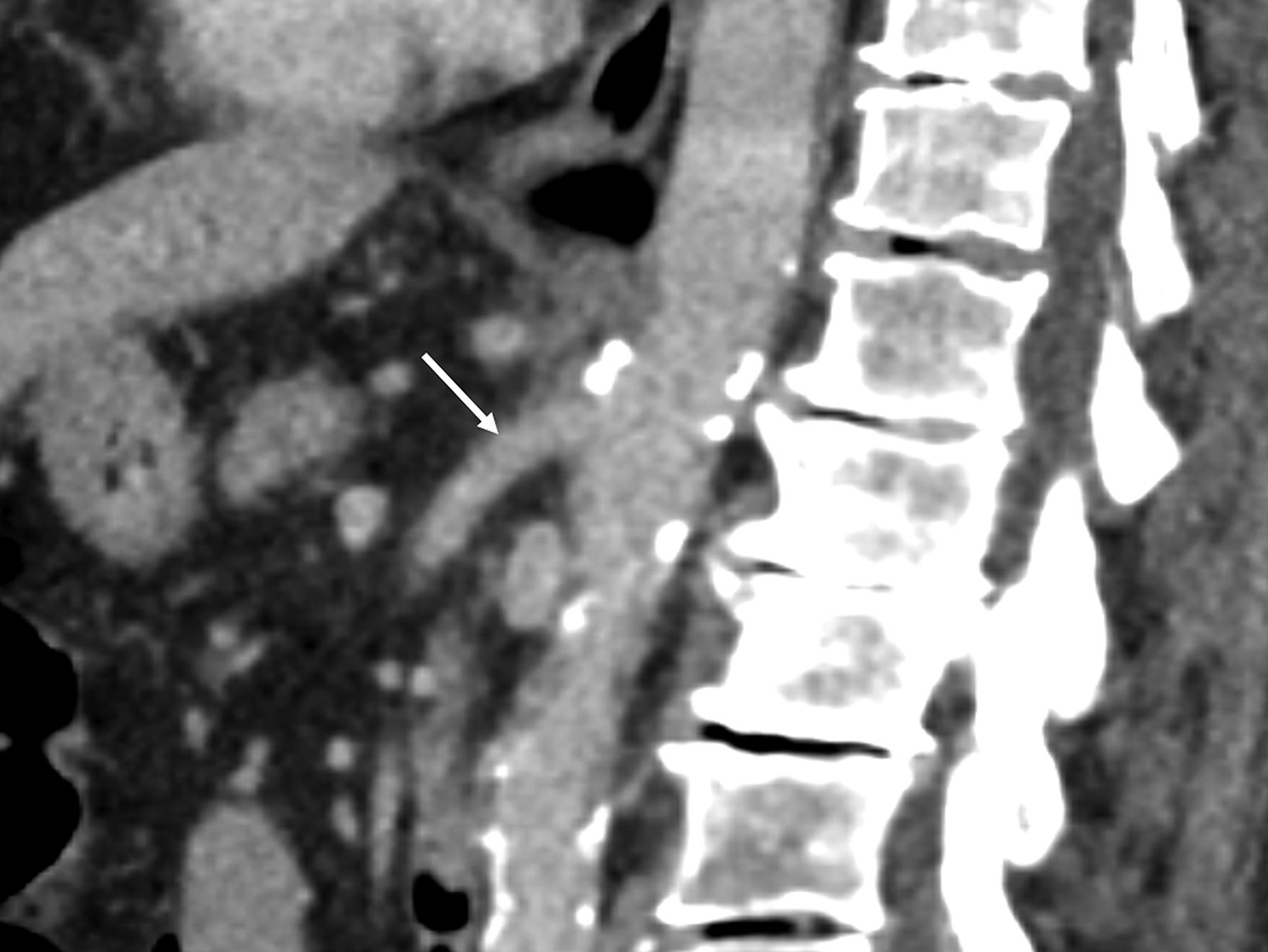
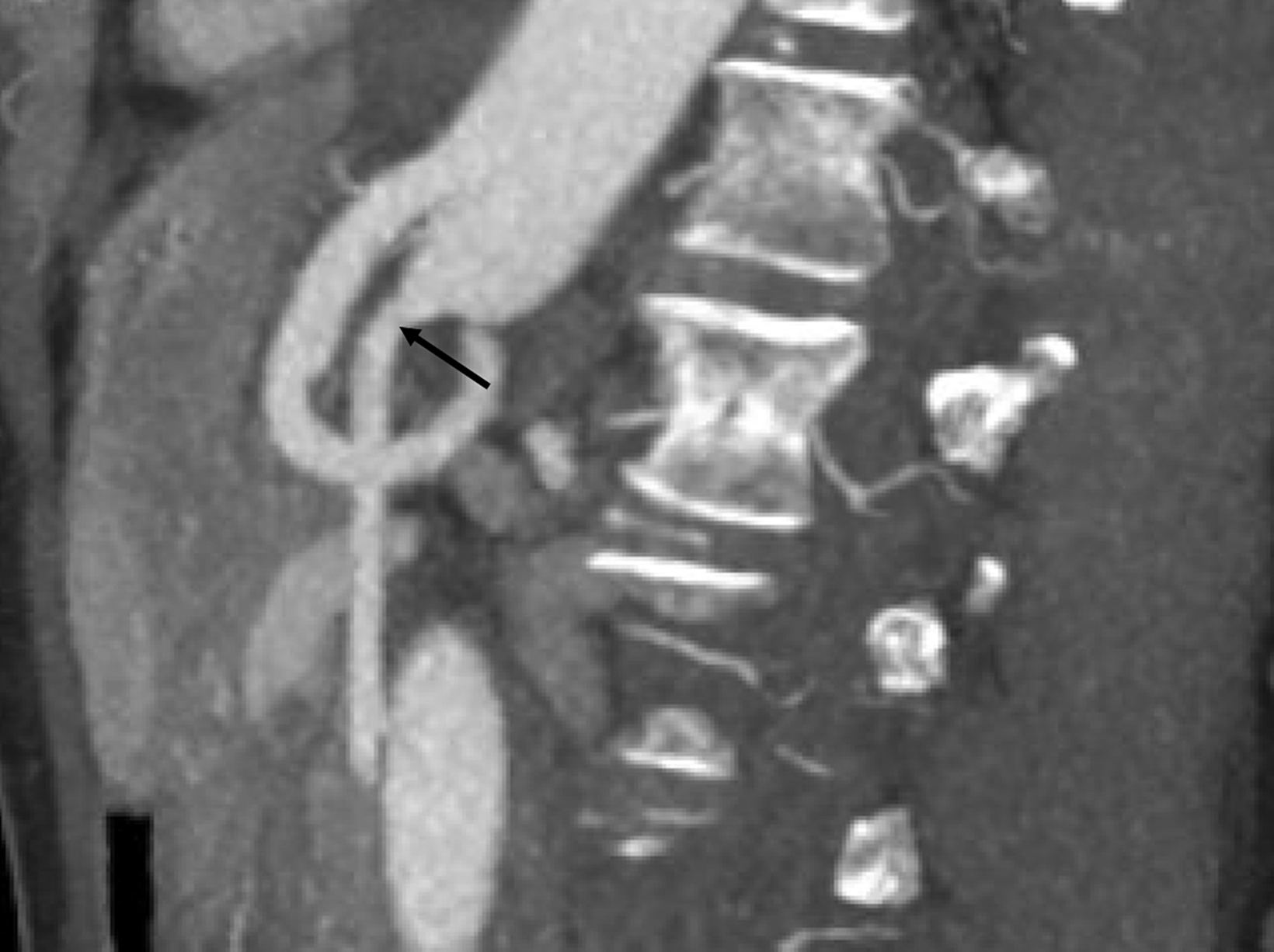
Sonographic evaluation of the mesenteric vessels has significant limitations regardless of fasting or postprandial state. These include patient body habitus, bowel gas, and anatomic variations, as well as variability between sonographers and interpreting radiologists. Overestimation of stenosis may occur secondary to variant anatomy, vessel tortuosity, and the presence of dense calcifications without significant luminal narrowing due to alterations in blood flow (Figure 7).1,3,13 Furthermore, the importance of clarity on the sonographer worksheet cannot be overstated to avoid confusion surrounding the interpretation of fasting and postprandial PSV, which may inadvertently lead to overdiagnosis of SMA stenosis (Figure 8).
Management of Chronic Mesenteric Ischemia
In cases of CMI diagnosed at sonography, the next step in management may consist of CTA for further characterization of the stenosis or catheter angiography for definitive diagnosis and treatment. Abdominopelvic CTA has been demonstrated superior to ultrasound at detecting high-grade stenosis of the celiac and mesenteric arteries.13 In fact, CTA and catheter angiography are now considered the gold standard in assessing CMI.14 The treatments of choice for CMI include endovascular interventions, such as balloon angioplasty and stent placement, and open surgical interventions such as endarterectomy and bypass.13,14 The latter interventions are typically reserved for symptomatic patients who are otherwise in relatively good health with a focal lesion.13,14 A meta-analysis of several published series evaluating the outcome of endovascular treatment of CMI found high clinical success rates (80-100%).1 Follow-up ultrasound and/or CTA is typically performed to assess for vessel and/or stent patency.3,13,14 Conservative management is usually pursued in patients with multiple medical comorbidities and generally consists of antiplatelet therapy, anticoagulation, and/or nitrate therapy.13,14
Conclusion
While postprandial duplex sonography of the mesenteric vessels may provide additional information regarding the functional reserve and collateral flow in patients with CMI, postprandial imaging in the mesenteric artery ultrasound protocol may result in confusion and overdiagnosis. This potential for overdiagnosis based on expected elevated postprandial PSV values may lead to unnecessary imaging and unnecessary interventions. Fasting duplex sonography has proven useful for CMI screening, although there are pitfalls pertaining to operator dependency and patient-related factors such as bowel gas, body habitus, variant anatomy, and vessel tortuosity.
References
Citation
KM Z, RS P, N M, TF T, B T, SM S, KK H.Chronic Mesenteric Ischemia: Mesenteric Artery Duplex Sonography and the Utility of Postprandial Imaging. Appl Radiol. 2024; (2):14-21.
March 5, 2024