Cardiac MDCT: Utility in delineating morphology and function
Images


Dr. Strub is currently a Radiologist at Mercy Hospital Anderson, Cincinnati, OH. He graduated summa cum laude from Saint Louis University, St. Louis, MO, in 1997 and graduated from The University of Cincinnati College of Medicine, in 2001. He completed his Diagnostic Radiology Residency in 2006 at the University of Cincinnati, where he served as Chief Resident. He has been actively involved in research and has authored or coauthored several abstracts, posters, and peer-reviewed articles.
With its increased availability, technical speed, improved postprocessing software, and ease of patient throughput, the applications for cardiac multidetector computed tomography (MDCT) continue to expand. The most widely publicized applications are for the characterization of the coronary arteries. However, data also exist to provide the clinician with a morphologic and functional cardiac analysis, extending its scope and utility as a noninvasive imaging modality.
With its increased availability, technical speed, improved postprocessing software, and ease of patient throughput, the applications for cardiac multidetector computed tomography (MDCT) are growing. One of the most widely publicized uses is for the evaluation of the patient with atypical chest pain, where it can provide exquisite detail of the coronary arteries to allow for the characterization of coronary artery plaque to help guide clinical decision making for the cardiologist and primary-care physician. Current imaging protocols also allow for the visualization of cardiac morphology, which is becoming more useful for atrial fibrillation (AF) treatment and for patients undergoing complex cardiothoracic surgery. Data also exist to predict clinical outcomes and monitor treatment response in patients.
Utility of visualizing morphology and its effects on treatment
Visualizing cardiac morphology, particularly of the left atrium (LA), is becoming increasingly important in patients with AF. Through multivariate analysis, the Framingham study showed that left atrial diameter is an independent risk factor for the subsequent development of AF, with a hazard ratio of 1.39 for every 5-mm increase in size. 1 Determining atrial size may help provide insight into the complications and sequelae of AF, 2 as studies have shown a relationship between atrial size and stroke, 3,4 with a stepwise increase in the rate of stroke for every 10-mm increase. 5 Furthermore, there is an opinion that the larger the LA, the less likelihood of remaining in sinus rhythm. 2 While the measurement of LA size by M-mode echocardiography is widely used, it is inaccurate because it provides only a single anteroposterior dimension 6 and the quality of the images is also dependant on the skill of the echocardiographic technician and on the body habitus of the patient. 6 Measurements of LA diameter can easily be obtained from MDCT images without the technical limitations faced by echocardiography.
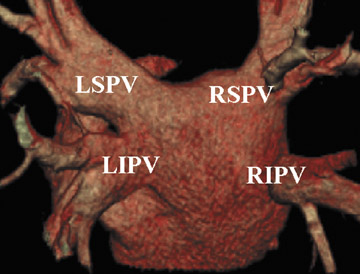
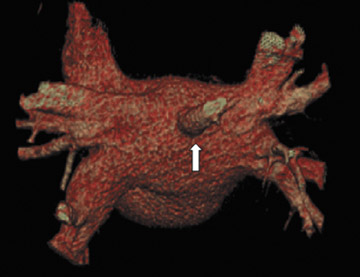
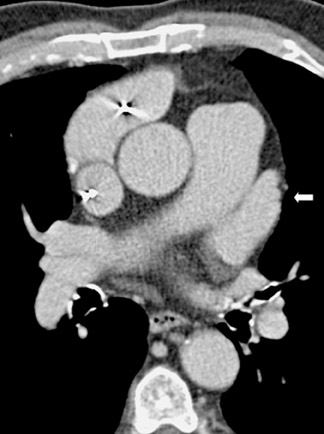
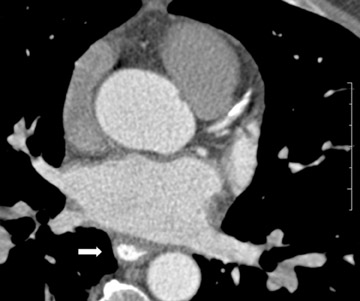
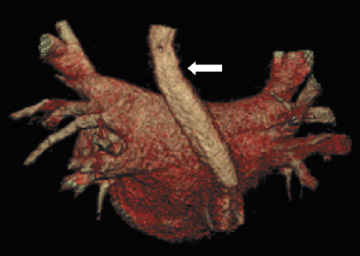
The applications of MDCT in preprocedural planning for the treatment of AF are expanding. Preoperative evaluation of patients prior to the Wolf mini-maze, 7 a minimally invasive thoracoscopic surgery for the treatment of AF, is one such example. 8 In addition to excising the left atrial appendage, this procedure involves isolating the pulmonary veins (Figure 1A), by placing a radiofrequency device on the atrium medial to the pulmonary veins. 7 In addition to detecting coronary artery anomalies, performing MDCT prior to the procedure can help detect several findings that can affect the surgical approach, such as atrial appendage thrombus, atypical course of the pulmonary veins (Figure 1B), and pre-existing pulmonary vein stenosis from prior ablation attempts (Figure 1C ).
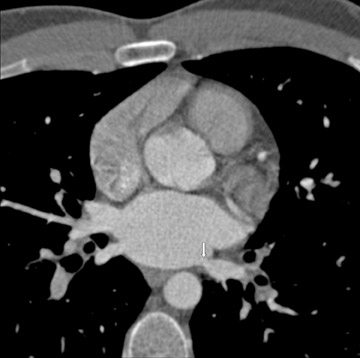
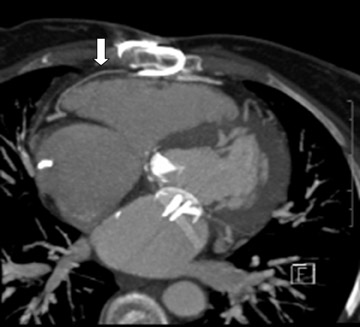
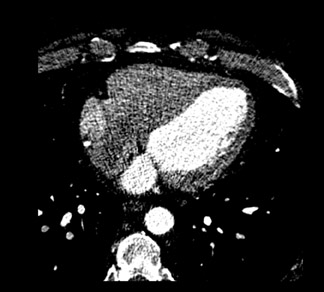
Evaluating the morphology of the left atrial appendage is also important, particularly as it relates to the detection of thrombus, as the presence of thrombus or sluggish flow can increase the risk of embolic stroke. 9 Transesophageal echocardiography (TEE) is the gold standard for the detection of thrombus, 10 but false-positive results occur with TEE. 11,12 Mohrs et al 13 concluded that magnetic resonance imaging (MRI) lacks diagnostic accuracy for the detection of a left atrial appendage thrombus. With MDCT, the difficulties are in distinguishing thrombus from sluggish flow (Figure 2) in the left atrial appendage, and this decreases its specificity. However, the addition of a 60-second delayed scan when performing MDCT can help improve the specificity of the examination for thrombus (C. Meyer, MD, unpublished data, February 2007).
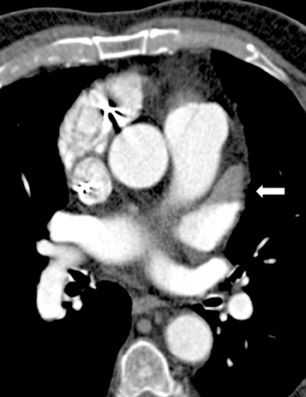
In addition to revealing the pulmonary vein anatomy and morphology of the LA, it is also important for the electrophysiologist to determine the location of the esophagus with respect to the LA prior to beginning a catheter-guided ablation procedure for AF. The course of the esophagus varies along the posterior LA atrium; however, Lemola et al 14 noted that in the majority of patients the esophagus is parallel to the ostia of the left-sided pulmonary veins and in direct contact with the posterior left atrium for >5 cm along its long axis. The authors emphasized that the target sites during catheter ablation of AF may often fall within this region of contact and suggested that the presence of a fat pad around the esophagus insulates it from thermal injury. Therefore, using CT to identify patients who do not have this fat pad prior to the procedure could be beneficial. l4 Understanding the spatial relationship (Figure 3) can avoid the potentially lethal complication of left atrial-esophageal fistula, which can result in death in up to 50% of cases. 15 However, the mobility of the esophagus may necessitate real-time imaging for an accurate awareness of the position of the esophagus during ablation procedures. 16
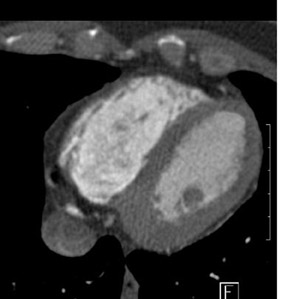
Evaluating the morphology of the coronary venous anatomy (Figure 4) is also of interest to the clinician. Knowledge of the coronary venous anatomy can help guide multiple therapies, including catheter placement in the coronary sinus for ablation procedures, 17 placement of left ventricular (LV) or biventricular pacing devices in patients with heart failure, 18,19 and the determination whether the venous system can be used to bypass coronary artery stenoses for percutaneous in situ coronary venous arterialization 20 or used for retroperfusion therapy. 21,22 The current gold standard for imaging coronary veins is retrograde venography via the coronary sinus. 23 However, recent reports have shown promising results for the use of MDCT angiography as a tool for the visualization of the coronary veins. 23,24
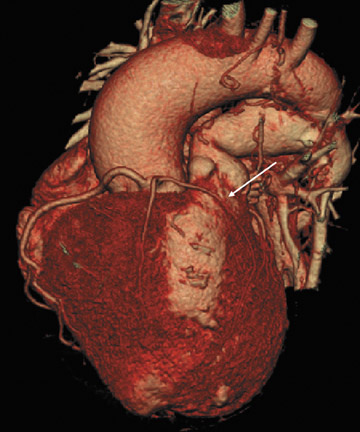
Clear delineation of cardiac morphology is also imperative for the cardiac surgeon in cases of repeat sternotomy. Elahi et al 25 examined the complication rate in 185 patients who underwent repeat sternotomy. They found complications directly attributable to repeat sternotomy in 21 (11.3%) of the cases. These included minor injuries to the aorta (4.8%), the right ventricle (RV) (3.2%), the right atrium (1.0%), prior bypass grafts (1.0%), and the right lung (0.5%). Most notably, there was uncontrollable hemorrhage from a tear in the aorta (0.5%) and 3 deaths (2.6%). The overall mortality in the repeat sternotomy group in the literature has been as high as 13.8%. 26
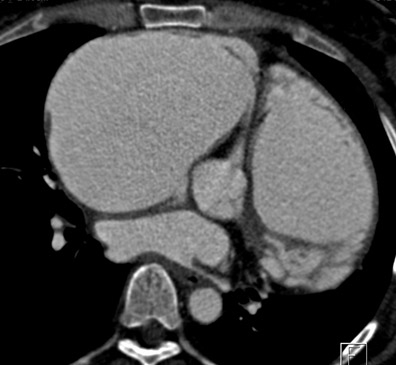
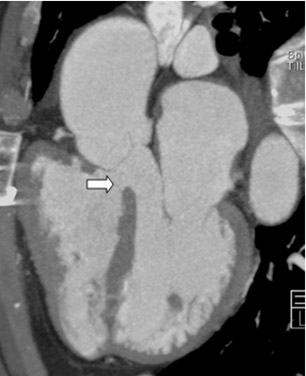
After undergoing sternotomy, the anterior mediastinal anatomy may be distorted by fibrosis. Major injuries may result in sternal re-entry due to inadvertent laceration of the heart, coronary arteries, or great vessels. Injury to implanted saphenous vein grafts has been reported and can result in massive intraoperative hemorrhage, myocardial ischemia, and infarction 27 (Figure 5). The lack of adequate retrosternal space is one of the main risk factors for sternal re-entry. 25,28 This is of particular importance in patients with congenital heart disease, such as Ebstein's anomaly (Figure 6).
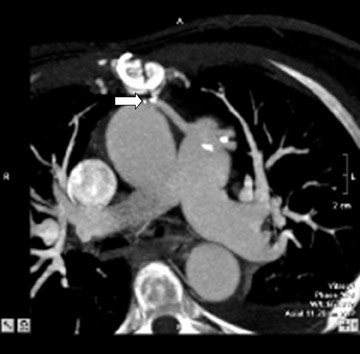
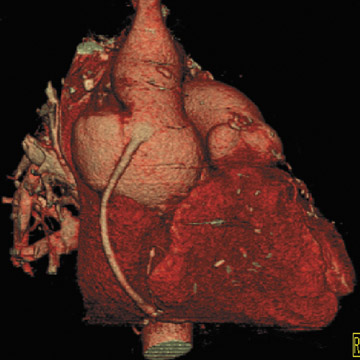
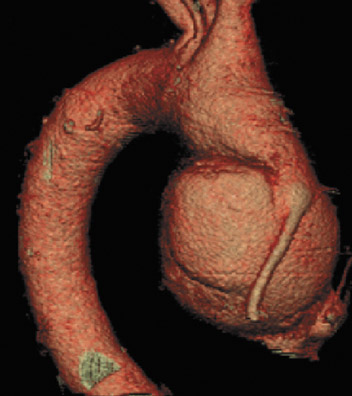
If there is increased risk of damage to retrosternal structures during sternotomy, the femoral vessels are commonly isolated prior to the procedure. Partial cardiopulmonary bypass using the femoral artery and vein is subsequently used to decompress the cardiac chambers and cool the patient (circulatory arrest) prior to sternal division. The presence of pseudoaneurysms (Figure 7) or the enlargement or anterior displacement of the RV may be indications for peripheral bypass. 27 In a study of patients who underwent preoperative cardiac MDCT for planning of complex adult heart surgery, surgical management was affected by the results of the CT scan in the majority of the cases. 29
In patients with congenital heart disease, detecting coronary artery anomalies prior to operative intervention is also imperative. Detecting congenital coronary artery anomalies can influence operative intervention in up to 60% of patients. 30 This is of particular importance in tetralogy of Fallot, in which anomalous coronary artery vessels can cross the RV outflow track and can interfere with its reconstruction. 31 Cardiac catheterization has always been considered the gold standard for the delineation of coronary anatomy prior to complex congenital heart surgery; however, Lee et al 28 recently reported the accuracy of MDCT in evaluating complex congenital heart disease in neonates and suggested that MDCT could replace diagnostic cardiac catheterization (Figure 8).
Assessing cardiac function
In patients with cardiac disorders, a desirable test to measure function is one that can provide quick, accurate, reproducible, and noninvasive high-quality images to determine the severity of cardiac impairment and evaluate the efficacy of treatment. 32 In patients with ischemic heart disease, the ejection fraction (EF) has been shown to be a more powerful predictive factor of clinical outcome than the number of vessels involved. 33,34 Currently, cardiac function can be measured with ventriculography, echocardiography, MRI, electrocardiographic (ECG)-gated single-photon-emission CT (SPECT), electron-beam CT, and MDCT.
Conventional ventriculography to measure cardiac function, while often considered the gold standard, is an expensive and invasive modality performed during angiography. However, it is limited by the geometric assumptions that are made from projectional images. This can lead to the inability to evaluate regions of the heart where there are complex irregular shape changes. 33
Transthoracic ECG (TTE) is widely used, as it can provide accurate measurements of ventricular volumes and mass as a cost-effective 33 bedside cardiac evaluation tool. However, TTE results are heavily technician-dependant, and in approximately 10% of patients, the endocardial borders cannot be well defined, in which case the functional assessment depends on the operator's experience and subjective visual perception. 35 The complex shape of the RV and its retro-sternal location also make functional evaluations of the RV particularly difficult by echocardiography. 36
With its multiplanar nature and high spatial and contrast resolution, MRI is likely the noninvasive standard of reference for the qualitative assessment of ventricular volumes, EF, and mass. 33,37 Other advantages of MRI include the lack of radiation exposure and the fact that it does not require injection of contrast media. 37 In addition to its increased cost, MRI does have limitations in that it cannot be performed in patients with implantable devices (such as pacemakers or defibrillators) and some MR sequences are susceptible to irregular or changing heart rates. 37 Prolonged examination times in the supine position and repeated breath-holds can be stressful for patients with breathing difficulties and can lead to degradation of image quality. 36
Electrocardiographic-gated SPECT offers the ability of combining 3-dimensional (3D) assessments of LV volumes and consecutively calculated function parameters. 32 Its accuracy is limited by its low spatial resolution, and the definition of endocardial borders in patients with thinning after infarction may be difficult because of low counts from these areas. 38,39 The need for repeated radionuclide doses is also problematic due to radiation exposure 37 ; therefore, it remains a projection method, which means that it provides less anatomic methods than competing modalities. 40
Electron-beam CT can provide a temporal resolution of 50 to 100 msec to produce a motion-free image of the heart in diastole, 41,42 but it is not widely available. However, the longitudinal resolution in the z-axis is limited, which can impair 3D visualization and ventricular volume calculation. 37
Multidetector CT can image the heart with high spatial and temporal resolution in a single breath-hold with ECG synchronization to provide high-quality 3D data sets for postprocessing that can be used to measure cardiac function 33 without the use of geometric assumptions. Retrospective ECG gating and cardiac software developments allow wall motion to be evaluated and functional calculations to be made. The MDCT images should be less susceptible to cardiac arrhythmias than MRI because of the retrospective gating with MDCT versus the prospective gating with MRI. 37 Since MDCT is a true volumetric modality, enlarged or grossly deformed hearts should not influence the accuracy of the measurements. 37
MDCT for the evaluation of cardiac function
Many functional measurements can be calculated after determining the end-diastolic volume (EDV) and end-systolic volume (ESV). The EF is defined as EF = EDV-ESV/EDV × 100% and has a normal range of 50% to 70%. The stroke volume (SV) is defined as SV= EDV-ESV, and cardiac output (CO) can be derived when the heart rate (HR) is considered CO = SV × HR. When the body surface area (BSA) is considered, advanced calculations can be determined, including the stroke index (SV/BSA) and the cardiac index (CO/BSA).
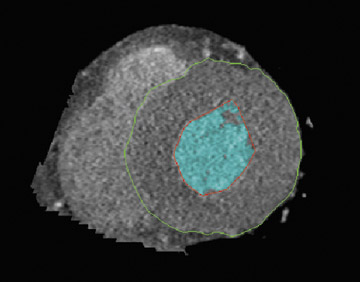
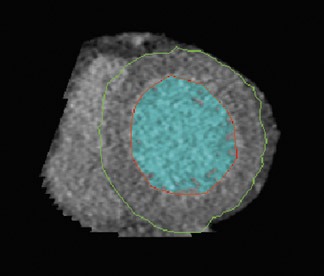
Like echocardiography and MRI, LV volume measurements are based on short-axis reformations using Simpson's method. A diastolic and systolic phase is needed for LV functional assessment, which is usually found at the minimum ventricular diameter (25% of the R-R interval) and the maximum ventricular diameter (85% of the R-R interval). 43 Diastolic and systolic volumes can be calculated using MDCT software in the end-diastolic and end-systolic phases. Epicardial and endocardial contours can be drawn either manually or automatically with cardiac software (Figure 9). Software packages can calculate ventricular volume and wall thickness to help speed analysis. Automated segmentation algorithms used in MDCT are sensitive to adequate contrast opacification, as the delineation of the trabeculae and thus the volume can be influenced by the degree of contrast opacification. 32 For accurate volume calculation, the papillary muscles should be excluded from the cav-ity. 33
The total electromechanical systole duration is approximately 300 msec, with the minimal ventricular volume being maintained for only 80 to 200 msec. 33 A temporal resolution of 30 to 50 msec per scan is necessary to image maximum systolic contraction. With the current repetition time of 64-slice MDCT at 165 msec, the volume changes during rapid ventricular filling and ejection are not well represented. While plain volume has excellent agreement with MRI, the regional functional analysis is limited, as there may be a slight overestimation of LV ESV by MDCT compared with MRI, which can result in an underestimation of LV EF from 1% to 7%. 37 However, the reproducibility of global functional parameters is in the range of other modalities and is close to that of MRI, which is accepted as the reference method for precise quantitative LV functional analysis for LV EDV and LV ESV. 44-46
The evaluation of RV function is also important, particularly as it relates to the outcomes of patients with acute pulmonary embolism, 47,48 since RV failure is the main cause of death within 30 days. 33 Assessing RV function is important in identifying high-risk patients so the appropriate therapy can be initiated. Also, RV function analysis 33 has implications in congenital heart diseases involving the RV and chronic heart failure. 49 Recently, it has been shown that RV systolic function can be accurately assessed using ECG-gated MDCT data 45 ; however, MRI is still accepted as the gold standard for measuring RV function. 50 When compared with MRI, MDCT offers a smaller variance in RV volumetric measurements. This is likely a direct result of the single breath-hold technique for MDCT rather than the multiple breath-hold MRI technique. Furthermore, estimates of RV EF calculated retrospectively from ECG-gated MDCT were found to be similar with scintigraphy and were accurate in patients with known or suspected RV dysfunction. 36 If functional parameters are to be measured, delineation of the right cardiac chambers is improved with a biphasic injection or with the administration of dilute contrast after the initial bolus injection 37 (Figure 10).
In addition to evaluating LV and RV fraction, the left atrial EF can also be assessed by measurements of the EDV and ESV. A recent report highlighted the importance of left atrial EF as it relates to left atrial transport function. Lemola et al 51 found that the restoration of sinus rhythm by left atrial catheter ablation compromised left atrial systolic function, which suggests that the loss of contractile function was likely related to the amount of tissue that was ablated. After catheter ablation therapy, the left atrial EF was similar in patients with paroxysmal AF and in those with chronic AF who were in sinus rhythm, but it was noted that the LA function in these 2 groups was lower than in control subjects with no history of AF.
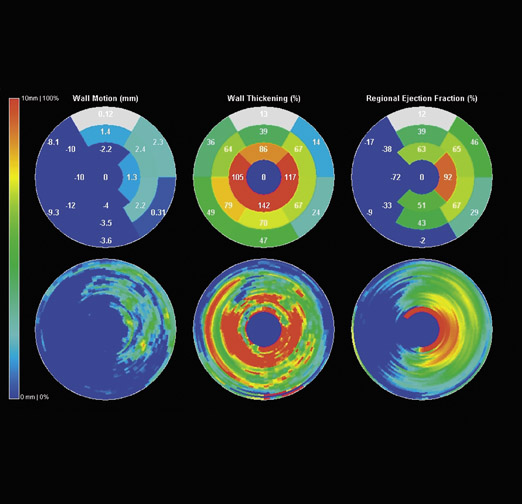
In addition to EF, a recent review defined the important parameters involved in assessing cardiac function. 33 Wall motion can be defined as the displacement of the points from the end-diastolic phase to the end-systolic phase. Wall thickening is indicative of the regional percentage of wall thickening during the systolic phase and is the single most accurate measurement of local function, since endocardial wall motion can be difficult to assess accurately in patients with ischemic heart disease. Regional EF expresses the EF relative to a specific region or segment of myocardium. A semi-quantitative estimation of these regional parameters can be performed using color polar maps. The bull's-eye pattern based on the 17segment approach of the American Heart Association should be used 52 (Figure 11).
If regional wall motion is to be displayed, at least 10 to 20 heart phases are needed. 37 Images can be viewed in a cine loop to qualitatively assess LV wall motion. Normal contraction requires functional tissue with adequate blood supply, and the reduction of blood flow below a critical threshold prevents normal contraction. 37
A hypokinetic segment is defined as impaired contraction, akinesis is when there is no motion, and dyskinesis is the paradoxical outward motion in systolic contraction. Generalized wall motion abnormalities occur in dilated cardiomyopathy and end-stage valvular heart disease; however, regional wall motion abnormalities occur in ischemic heart disease. 33
Functional analysis requires that no medication be administered before the MDCT examination that could influence the patient's heart, since an artificially reduced heart rate may alter the functional parameters. The use of dual-source CT may potentially diminish the need for beta-blockers and improve volume and EF measures. 32
Short-axis images are readily available in MRI, and time-consuming secondary cardiac reformations in cardiac MDCT are not required. The total evaluation time of MDCT functional analysis has become the focus of recent studies, and reported evaluation times range from 30 to 50 minutes for 4-channel MDCT 53 and 14 to 20 minutes for 16-channel MDCT. 54
Costs of evaluating cardiac morphology and function
The cost effectiveness of the assessment of cardiac morphology and function with examinations must also be considered. For fiscal year 2007, our hospital charges for the examinations are as follows (CPT codes noted): $2821 for a cardiac MRI with contrast (75553), $2166 for a cardiac CT without calcium score (0148T/76497), $812 for 2-dimensional TTE (93307), and $2025 for a myocardial perfusion SPECT scan (78465).
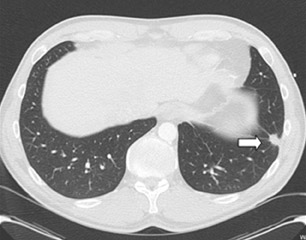
While the cost of cardiac MDCT is high, and there is a trade-off of radiation dose, it offers the benefit to the patient of detecting other abnormalities in the thorax that would not be seen by other modalities (Figure 12). Extracardiac findings can be detected in up to 24% of patients, and nearly 5% of these may be major findings such as lung carcinoma and pulmonary emboli. 55
Conclusion
Cardiac MDCT is a valuable clinical tool for the evaluation of cardiac morphology and function. In patients with atrial fibrillation or those undergoing open heart surgery, it is a valuable tool that can clearly delineate the relationship of native coronary arteries, coronary artery bypass grafts, and the cardiac chambers to the sternum and mediastinal structures. Armed with this spatial knowledge, the surgical approach may be altered to avoid potential complications related to prior surgery, chamber dilation, or mass effect. While it is not the first-line modality for evaluating cardiac function, MDCT can also evaluate cardiac function and wall motion in conjunction with a CT angiogram, and it offers the added benefit of detecting clinically significant abnormalities in the thorax. Therefore, the clinician can derive a great deal of accurate diagnostic and clinically useful information in a study that can be performed quickly.
Acknowledgments
The author would like to thank Rhonda Strunk, RT(R)(CT) and Cristoper Meyer, MD, for their help and support.