Calcific Subcoracoid Bursitis
Case Summary
An elderly patient presented to their orthopedic surgeon complaining of right shoulder pain. The patient had been experiencing discomfort localized to the anterior and superior shoulder for the past 2 months, with no preceding history of trauma. The patient’s medical history included obstructive sleep apnea, laryngopharyngeal reflux disease, chronic rhinitis, hypertension, and hyperlipidemia. Upon physical examination, tenderness was noted over the anterior shoulder and the acromioclavicular joint, along with positive impingement signs and a positive cross-over test. In response, the patient received intra-articular glenohumeral and subacromial-subdeltoid bursal injections containing a steroid and an anesthetic. The patient was advised to engage in gentle range-of-motion exercises as tolerated and to take nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen for pain management as needed. Following 2 weeks without significant improvement in their symptoms, a prednisone taper was prescribed. Although the patient reported some relief, their pain persisted.
Imaging Findings
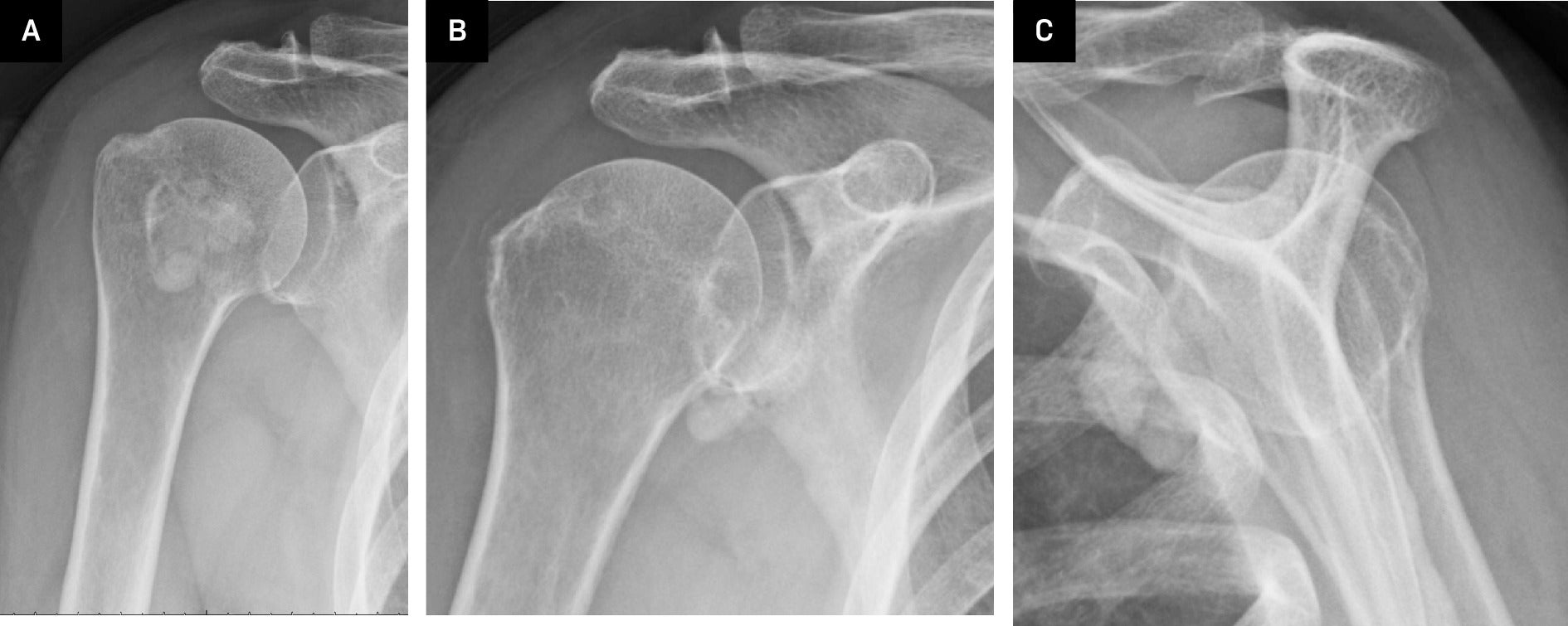
Right shoulder radiographs revealed a 2.8 × 3.4 × 1.7 cm (medial-lateral × craniocaudal × anteroposterior) calcified macro-lobulated density anterior to the humeral head in a subcoracoid position on the scapular Y view ( Figure 1 ). Notably, the calcification appeared denser in the inferior dependent portion. Non-contrast MRI depicted a uniformly mild T2 hyperintense and proton density isointense lesion, relative to skeletal muscle, with layering of hypointense material in the posteroinferior dependent portion, corresponding to the denser calcification observed on radiographs ( Figure 2 ). The lesion resided in a subcoracoid position, anterior to the subscapularis muscle, and extended between the short and long head biceps tendons, within the subcoracoid bursa. These findings were consistent with the location of the multilobulated calcified mass identified on the prior X-ray. Subsequently, an MRI with and without contrast revealed peripheral enhancement around the synovial lining of the distended subcoracoid but no internal enhancement ( Figure 3 ).
Radiographs of the right shoulder: Internal rotation (A), external rotation (B), and scapular Y-view (C) demonstrated multilobulated calcifications adjacent to the glenohumeral joint.
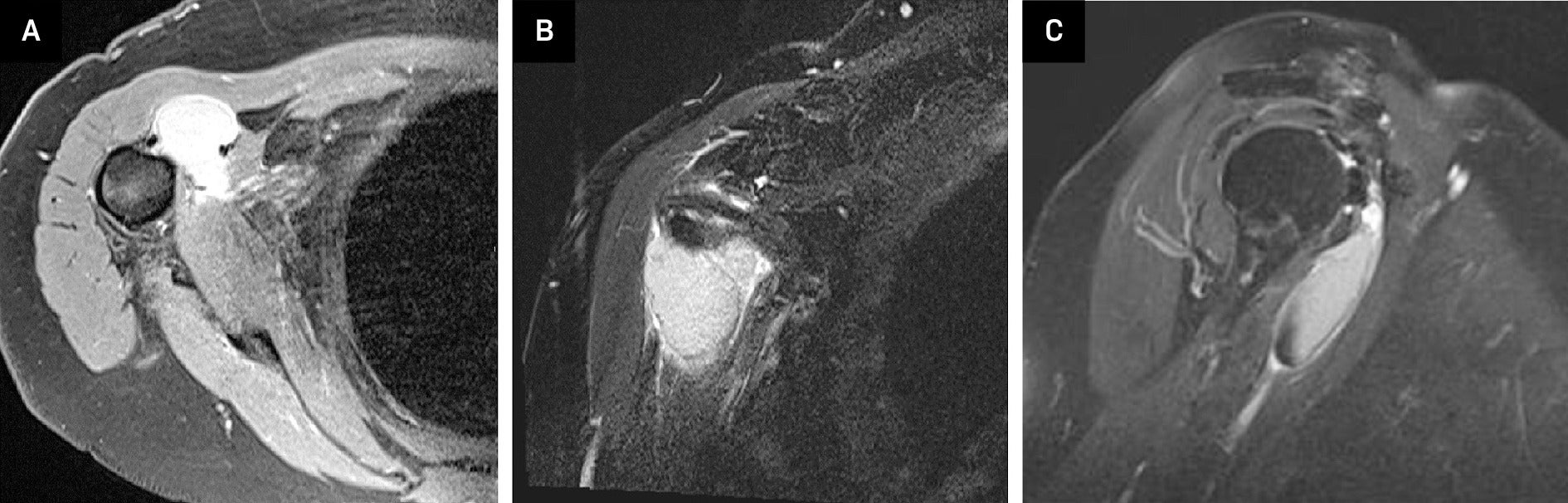
MRI shoulder without contrast: Axial view (A) of a proton density fast-spin echo sequence and a coronal view T2 fat-suppressed image (B) showed a 1.5 × 3.4 × 4.1 cm lesion which was hyperintense compared to skeletal muscle, but hypointense compared to fluid, located in a subcoracoid position. Sagittal view (C) showed a mild T2 hypointense signal along the inferior aspect of this lesion.
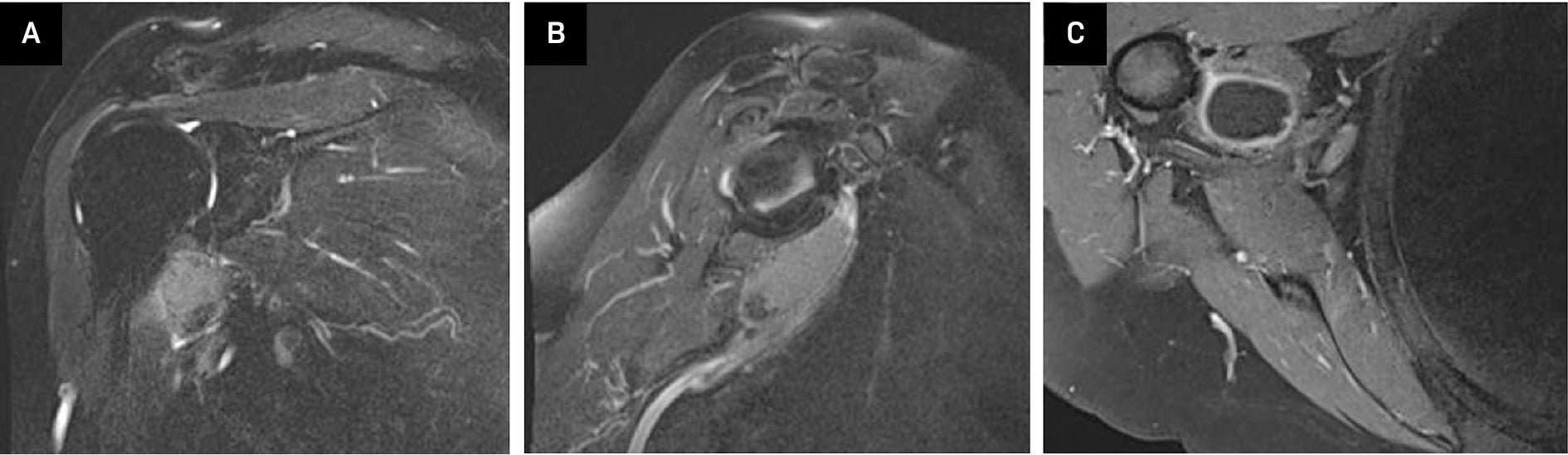
MRI with and without contrast: Coronal view (A) and sagittal (B) T2 fat-suppressed image without contrast again showed a hyperintense lesion within the subcoracoid bursa. A T1 axial fat-suppressed post-contrast image (C) showed peripheral enhancement around the synovial lining without internal enhancement.
Diagnosis
Calcific subcoracoid bursitis.
Discussion
Hydroxyapatite crystal deposition disease (HADD) is a crystal-induced syndrome characterized by the accumulation of hydroxyapatite crystals within joint spaces, tendons, or bursae. The pathogenesis of calcific tendinopathy, a hallmark of HADD, is attributed to decreased oxygen tension within tendons, prompting a phenotypic shift of tenocytes to chondrocytes and subsequent calcification.1 While HADD predominantly affects the shoulder, particularly the rotator cuff tendons, instances involving the subcoracoid bursa are rare. The subcoracoid bursa, delineated superiorly by the coracoid process and tendons of the short head of the biceps and coracobrachialis, with inferior borders formed by the subscapularis tendon, infrequently exhibits involvement in HADD.2
Conventional radiography typically reveals the characteristic appearance of HADD as a globular, homogeneous hyperdensity with smooth or indistinct margins. However, ultrasound offers a more dynamic assessment, showcasing calcific tendinopathy in various stages of calcification. These stages encompass precalcific, calcific, and postcalcific phases.3 Within the calcific stage, differentiation is made between a resting phase, marked by solid calcifications resulting in complete acoustic shadowing on ultrasound, and a resorptive phase, where softer calcifications lead to partial or absent shadowing.3 MRI further elucidates the pathology, depicting calcific tendinosis or bursitis as a T2 hypointense, homogeneous ovoid signal, often accompanied by variable hyperintense peripheral signals. These peripheral hyperintensities may denote concurrent edema or bursal fluid accumulation.4
During the resorptive phase of HADD, the migration of softer crystals to adjacent tissues via delaminating zones poses significant clinical challenges.3 Patients afflicted with this condition, frequently women with a median age ranging from 31 to 40 years,4 commonly present with symptoms such as joint swelling, diminished range of motion, and instability, often accompanied by episodes of acute shoulder pain. The resorptive phase exacerbates symptoms, particularly during movement, leading to pronounced discomfort. In such cases, interventions aimed at pain management become imperative. Under ultrasound guidance, the orthopedic aspirated our patient’s bursa, resulting in the extraction of 10 mL of tan chalky fluid from the affected area. The aspirated fluid was subsequently sent for crystal analysis. Ultrasound imaging confirmed successful decompression of the bursa. Ultrasound-guided corticosteroid injections or needling/lavage of the affected bursa are frequently employed to alleviate pain and restore functionality,3 mirroring the approach undertaken in our patient’s case.
HADD can manifest acutely, precipitating erosive changes in surrounding osseous structures and precipitating destructive shoulder arthropathy, exemplified by conditions like Milwaukee shoulder syndrome, attributed to hydroxyapatite crystal deposition. Furthermore, intraosseous calcifications may ensue, potentially manifesting as lytic lesions within the shoulder region.
Distinguishing HADD from other systemic diseases marked by intra-articular calcifications, such as calcium pyrophosphate deposition disease (CPPD) and gout, is imperative. While CPPD calcifications typically exhibit a more linear and diffuse pattern, gout can present with MRI findings akin to those observed in HADD.4 Notably, patients afflicted by gout often present with elevated urate levels, yet both gout and hydroxyapatite crystals exhibit negative birefringence under polarized light.4
It is imperative to discern HADD from more aggressive pathologies, as migration of hydroxyapatite crystals may lead to pronounced osseous changes characterized by cortical erosion, periosteal reaction, intramedullary edema, and soft tissue calcifications.1 Key differentiating features favoring a diagnosis of HADD over a more aggressive entity include the absence of joint effusion or soft tissue mass.1 Additionally, during the acute phase, HADD may exhibit MRI findings resembling those of infectious entities, typified by an extraosseous T1 hypointense rim and T2 hyperintense signal.1
Conclusion
While calcific bursitis frequently involves the supraspinatus tendon, the predominant site for calcific tendinopathy, our case report highlights a rare occurrence of calcific subcoracoid bursitis. Understanding the diverse imaging characteristics of this benign condition is paramount in preventing unwarranted interventions and facilitating timely and suitable symptom alleviation for patients. By enhancing awareness of such atypical presentations, clinicians can optimize diagnostic accuracy and therapeutic interventions, ultimately improving patient outcomes. Continued research into the nuanced manifestations of calcific bursitis is essential for refining diagnostic protocols and advancing management strategies in clinical practice.
References
Citation
Kalra R, Malik S, Abulencia AE, Dayan E.Calcific Subcoracoid Bursitis. Appl Radiol. 2025;
doi:10.37549/AR-D-25-0112
October 1, 2025