Bone tumors and tumor-like conditions of bone
Images










Dr. Cronin is a Resident Physician in the Department of Radiology, and Dr. Hughes is a Professor of Radiology, Residency Program Director, Department of Radiology, University of California San Diego, San Diego, CA.
Bone tumors are a relatively infrequent finding in musculoskeletal radiology. When evaluating osseous lesions, the radiologist’s main goal is to assess whether the lesion is benign or aggressive in appearance and whether further workup is required. The list of potential osseous lesions is extensive; this review of bone tumors does not include metabolic or degenerative lesions. To provide a meaningful differential diagnosis to the referring clinician, several characteristics of every osseous lesion should be routinely assessed.
Classically, 10 radiographic features of a bone lesion should be examined. Five of these include: zone of transition (ZOT), presence or absence of periostitis, location in the bone, pattern of osseous destruction, and age of the patient, along with associated symptoms.1-3 Additionally, determining if the process is mono-ostotic or polyostotic can be very helpful. Because these characteristics are so important to properly classifying bone tumors, more should be said about each.
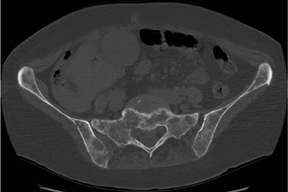
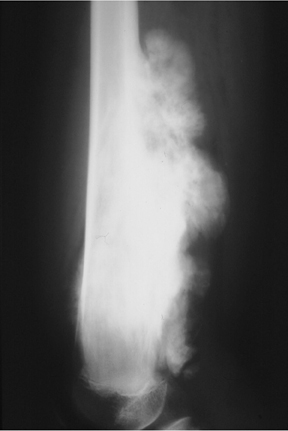
The zone of transition is that outer margin of the lesion that represents the change from pathologic to normal bone. A wide ZOT is said to be present when the lesion cannot be clearly circumscribed; this is usually associated with an aggressive lesion. Malignancies such as Ewing’s sarcoma and osteosarcoma typically show this pattern of involvement; however, infection and other benign processes, such as eosinophilic granulomas (EG) (Figure 1), can have wide margins. A wide ZOT does not equate to malignancy, but it is very rare for a narrow ZOT (a geographic lesion) to be associated with anything other than a benign lesion.3,4
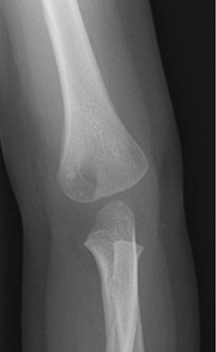
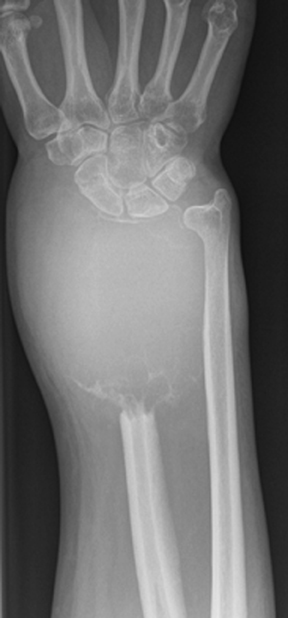
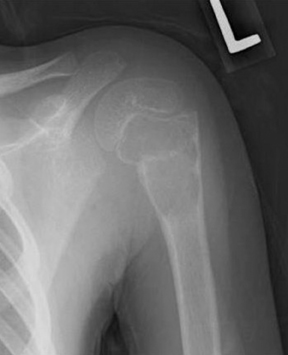
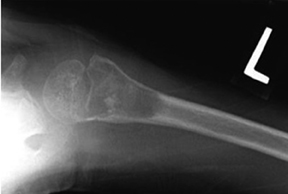
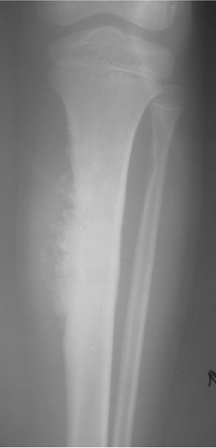
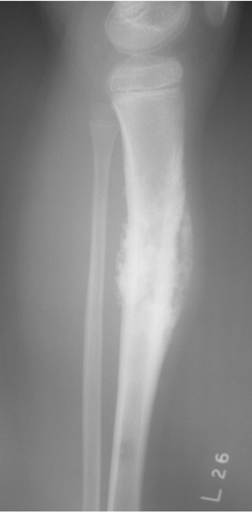
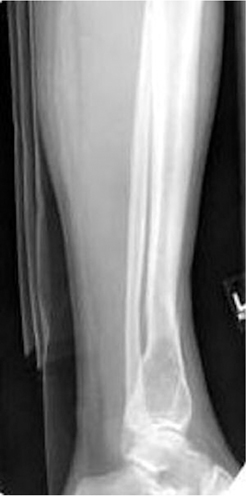
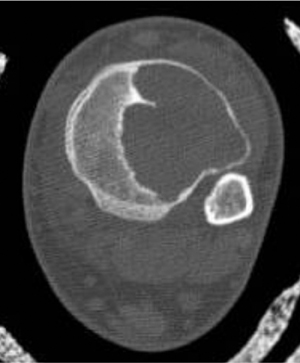
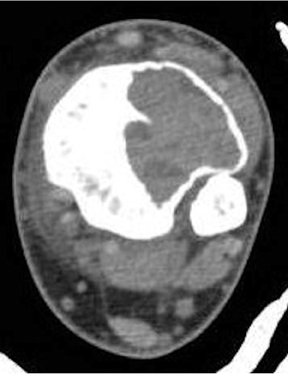
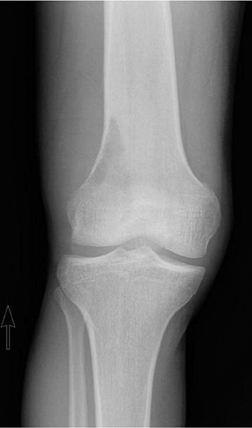
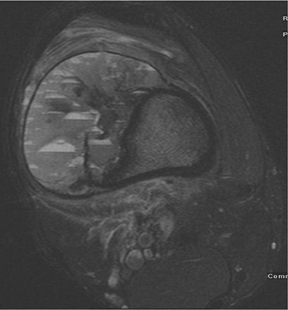
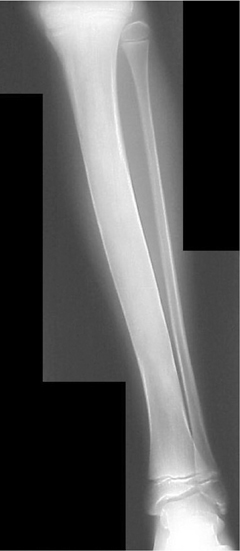
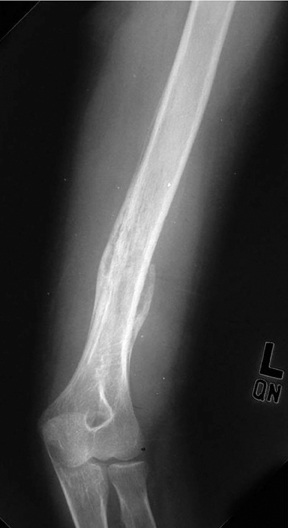
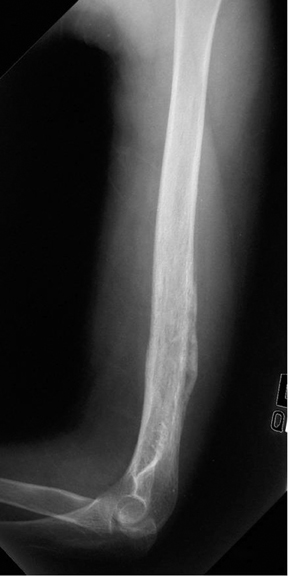
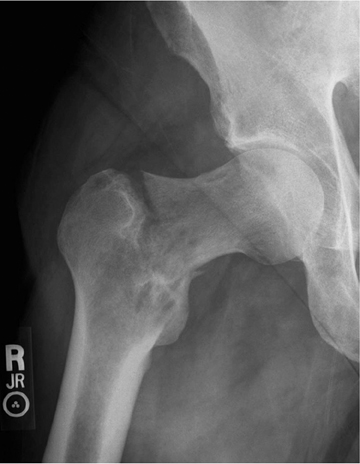
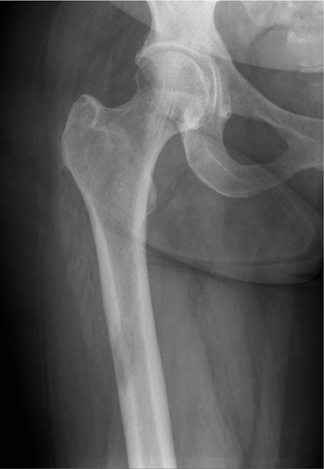
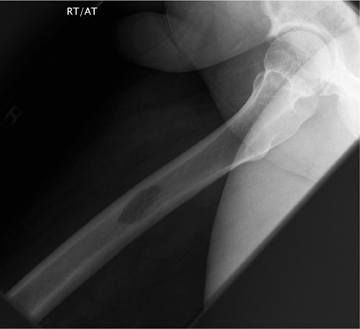
The location of the lesion in the bone, both transversely and longitudinally, can also be useful in narrowing the differential. For example, a select group of lesions is eccentrically located and involves the cortex (osteoid osteoma, parosteal osteosarcoma, and nonossifying fibroma) (Figure 2). Other lesions almost always involve the epiphysis (giant cell tumor after physeal closure (Figure 3, DICOM images available: 3A, 3B, and 3C, courtesy of Claron Technologies), chondroblastoma). Some lesions, such as solitary bone cysts (Figure 4), enchondromas (Figure 5), EG and Ewing’s sarcoma, tend to be centrally located.1,3
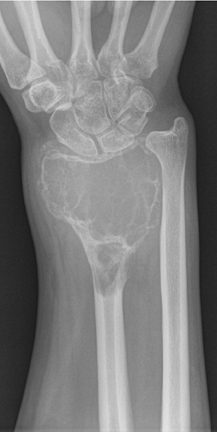
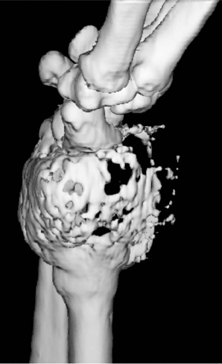
The number of osseous lesions can also provide clues to etiology. Several lesions that can be polyostotic include multiple hereditary exostoses, enchondromas, fibrous dysplasia and, occasionally, eosinophilic granulomas.3 Of course, metastatic disease and multiple myeloma (Figure 6) are common causes of multiple lesions, but a few bony metastases can present as solitary lesions—especially in renal or thyroid carcinoma.3,5
Elucidating information from periosteal changes can be relatively more difficult. Periostitis is often subtle and can mislead the radiologist attempting to classify a lesion as benign or aggressive. Classic, aggressive-appearing periostitis is described as having an “onion-skin,” “sunburst” (Figure 7), or “hair-on-end” appearance.2,3 A Codman triangle pattern is another aggressive configuration. Benign patterns are those that, in theory, have had sufficient time to organize and, thus, show solid thick or wavy unilamellar periosteal changes. Use caution when assessing periosteal reactions, as many benign lesions such as infection, EG (Figure 1), and aneurysmal bone cysts can result in an aggressive-appearing periostitis. Regardless, recognizing periosteal reaction of any type remains important, as this effectively excludes several lesions from the differential. If periostitis is present, fibrous dysplasia, solitary bone cyst, nonossifying fibromas, and enchondromas can be removed from consideration unless complicated by fracture.3,4
Two additional pieces of information that can be extremely helpful are the age of the patient and the presence or absence of pain. Some lesions, such as Ewing’s sarcoma and primary osteosarcoma, are seen overwhelmingly only in the pediatric, adolescent, and young adult populations. Likewise, EG, chondroblastoma, solitary and aneurysmal bone cysts are rarely seen in adults >30 yrs. Moreover, while infection, metastatic lesions, and aneurysmal bone cysts typically present with pain, discomfort is rare (in the absence of trauma) with fibrous dysplasia, enchondromas, and solitary bone cysts. Several other characteristics, such as the presence of sclerotic margins, soft tissue involvement, a pattern of bony destruction, endosteal scalloping, and the pattern of matrix calcification, can also aid in diagnosis.
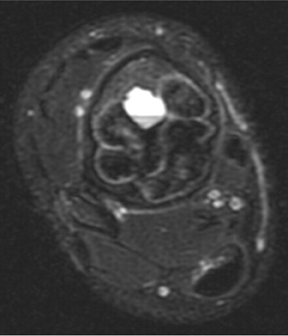
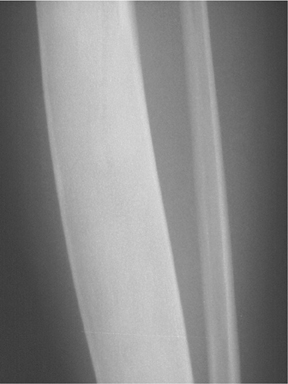
Although plain radiographs, computed tomography (CT), magnetic resonance imaging (MRI), and radionuclide studies may each provide additional information, suspected soft-tissue extension of an osseous lesion should be evaluated further with contrast-enhanced MRI to determine not only tumoral extent but also the risk of complications like neurovascular compromise. Also, MRI may help narrow the list of differential considerations by demonstrating cystic or necrotic components, encapsulation, contrast enhancement and the presence of fluid levels (Figures 2, 8, 9) or peritumoral edema on MRI. Dedicated CT may show occult, pathologic fracture in an otherwise benign-appearing but painful lesion.
Scintigraphy with Tc99m –MDP bone scan can determine whether a lesion is mono-ostotic versus polyostotic in nature. The following is a brief description of each of the 15 most common benign and malignant osseous lesions the radiologist is most likely to encounter.
Benign bone lesions
Solitary bone cysts
As mentioned previously, solitary bone cysts tend to be centrally located, painless lesions occurring almost exclusively in patients <30 yrs These tumors appear lytic, have a narrow ZOT and are most often located in the proximal humerus and femur (Figure 4). There is no periostitis unless an associated fracture is present. A fracture through an SBC may show the classic “fallen fragment” sign, which is fractured bone that has fallen to the most dependent portion of the cyst.3,4 As expected for a cystic structure, the MRI appearance of these lesions shows hypointense T1 and hyperintense T2 characteristics.
Aneurysmal bone cysts (ABC)
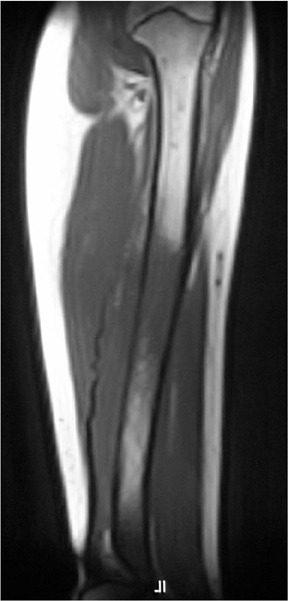
These metaphyseal lesions are seen almost exclusively in patients <30 years of age. In contrast to a unicameral or solitary bone cyst, an ABC is typically painful, can have periostitis (often aggressive) and is eccentrically located. The ZOT is usually narrow and an expansile bubbly appearance is common (Figures 2, 8). An ABC should be considered in the differential (along with osteoblastoma, tuberculosis, and osteoid osteoma) for lesions in the posterior elements of the spine. ABC often complicates other lesions, most notably chondroblastoma, osteoblastoma and giant cell tumor of bone. The MR and CT appearances may show fluid-fluid levels, which is a nonspecific finding and can be seen in other lesions, including chondroblastoma, giant cell tumor of bone, or telangiectatic osteosarcoma.3,6-9
Fibrous dysplasia
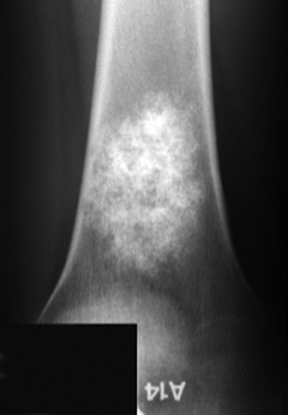
This painless, typically metadiaphyseal lesion can be monoostotic or polyostotic. When polyostotic, fibrous dysplasia is overwhelmingly (90%) located on one side of the body.3 The ZOT is narrow and the lesion itself can be quite heterogeneous with a lytic, sclerotic, or mixed appearance (Figure 10). When located in the pelvis, fibrous dysplasia can often appear lytic and bubbly while rib lesions may have a ground-glass appearance and be expansile. Involved tubular bones are expanded and demonstrate cortical thinning. In the hip, this can lead to varus angulation and a “Shepherd’s crook” deformity of the femoral neck.3,6,8,10 The skull base and calvarium are also common sites of involvement. There is no periostitis and the age range of affected patients is broad. The MRI appearance is nonspecific, typically showing T1 prolongation and variable T2 intensity.
Nonossifying fibroma
This common lesion is cortically based and eccentrically located. Found in individuals between 2 and 30 years, the painless entity shows a narrow ZOT with sclerotic borders and an appearance that can range from lytic initially to entirely sclerotic in the latter stages of development, as they typically “heal” and appear ossified (Figure 2). There is no associated periostitis. The MR appearance is nonspecific, with low signal on T1-weighted images and variable T2 signal.3,6–8
Giant cell tumor
This geographic, lytic lesion is classically described as having 4 qualifying characteristics when found in the long bones. It is eccentrically located, has a sharp nonsclerotic ZOT, abuts subchondral bone, and almost always occurs in individuals with recently closed physes (Figure 3). The most common locations are distal femur, proximal tibia and distal radius. However, atypical locations such as the pelvis and calcaneus are also seen.3,6,7,9,11 In these cases, giant cell tumors need not conform to the above criteria. These lesions are usually mono-ostotic; however, polyostotic tumors or satellite lesions are rarely seen and can be difficult to differentiate from metastatic GCT. Periostitis is not present. There is low signal on T1 and variable T2 MRI signal, which may include hyperintense regions or fluid levels secondary to aneurysmal bone cysts.
Eosinophilic granuloma
Eosinophilic granuloma (EG) could also be called the great mimicker of osseous lesions. While EG typically is found only in patients<30 years, there are a few other discriminating characteristics. For example, EG may appear lytic, sclerotic, mixed or “moth-eaten” (Figure 1). The ZOT can be narrow or wide. Associated periostitis may cause this lesion to appear aggressive. Infrequently, a bony sequestrum may be present; however, this can also be seen in osteomyelitis, fibrosarcoma and primary lymphoma of bone.3, 6, 8,12 Most EG lesions are monoostotic, but younger patients are at higher risk for developing polyostotic disease.
Enchondroma
Enchondromas are centrally located, geographic, and predominantly lytic-appearing lesions, which almost invariably contain a chondroid matrix when found in the long bones (Figure 5). These monoostotic, painless lesions may show endosteal scalloping; however, there is no associated periostitis. Enchondromas may be difficult to discriminate from low-grade chondrosarcomas, but the latter is more likely in a history of pain, cortical destruction or soft tissue extension.13 Large or proximal lesions have an increased rate of malignant transformation. In contrast, enchondromas found in the hands and feet may be polyostotic, but they rarely undergo malignant transformation except in Ollier’s disease or associated with soft-tissue hemangiomas, as in Maffucci’s syndrome.3,6 Chondroid matrix is conspicuously absent in these peripheral lesions.
Osteochondroma
This lesion arises from the metaphyseal region of the long bones as a bony excrescence contiguous with the medullary compartment that characteristically points away from the adjacent joint. Osteochondromas are typically solitary, appendicular lesions, which cease growing after the skeleton matures (Figure 11). Growth after this point, cortical erosion, or thickening of the associated cartilaginous cap is rare, but suggestive of malignant transformation into chondrosarcoma.3,6,14,15 Multiple hereditary exostoses are a rare, autosomal dominant, polyostotic form of the disease that has an increased risk of malignant transformation.
Malignant bone lesions
Osteosarcoma
In patients <30 years, osteosarcoma and Ewing’s sarcoma are the most common primary malignant tumors of bone. Osteosarcoma can be subdivided into medullary, parosteal, periosteal and telangiectatic, all of which carry different prognoses and treatment options.
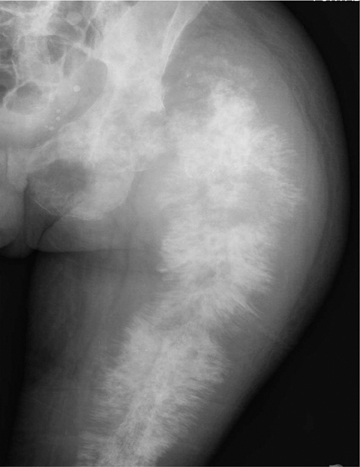
Medullary osteosarcoma (Figure 7) is the most common, occurring in adolescents and young adults typically between the ages of 15 and 25 yrs. These are rapidly growing, heterogeneous, metaphyseal lesions that show cortical destruction, aggressive periostitis, and a wide ZOT. Osseous matrix formation is the norm and a soft tissue component is often present. Evaluation with MRI shows variable T1 and T2 signal characteristics secondary to varying degrees of ossification. High T2 signal is often seen around the periphery of the lesion, presumably related to adjacent edema. Metastatic skip lesions are commonly seen and should be worked up with radionuclide evaluation.
Parosteal osteosarcoma (Figure 12) originates from the parosteal soft tissues and is less aggressive than the medullary form. This lesion typically occurs in older patients. Growth is circumferential around the involved bone and typically does not initially result in cortical destruction. As such the prognosis for this lesion is more favorable. However, if intramedullary extension does occur, the prognosis is more guarded.
Telangiectatic osteosarcoma (Figure 9) has the potential to be misdiagnosed as a giant cell tumor or chondroblastoma, as it is characteristically lytic and deceivingly nonaggressive in appearance. Subtle cortical erosion or periostitis may be present. Periosteal osteosarcomas are rarer than those mentioned previously. Periosteal osteosarcoma is a surface lesion that typically produces a sunburst or Codman’s triangle periostitis. Adjacent cortical erosion is usually seen.3,4,7
Ewing’s sarcoma
Ewing’s sarcoma is the most prevalent primary malignancy of bone in children and is second to osteosarcoma in adolescents and young adults. Classically, Ewing’s is described as a diaphyseal-based, permeative lesion with a wide ZOT and aggressive periostitis (Figure 13). An associated soft tissue mass is invariably present. It should be noted, however, that Ewing’s is not limited to a diaphyseal location; in fact , it is often found in metaphyseal or metadiaphyseal locations. This lesion typically affects the appendicular skeleton in children but is found more axially in adolescents. The radiographic appearance of Ewing’s is nonspecific and can overlap that seen with infection or eosinophilic granuloma. MRI characteristics show low signal on T1, high signal on T2, and typically an adjacent soft-tissue mass that may be heterogeneous in appearance secondary to focal necrosis.3,4,7
Chondrosarcoma
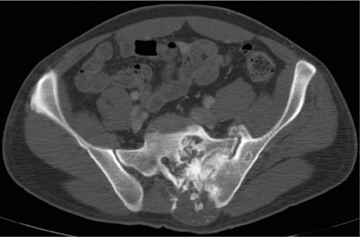
Chondrosarcoma is typically a low-grade malignant osseous tumor found in adults. The radiographic appearance can be misleading, as often few aggressive features are present. This is a lytic, metaphyseal-based lesion, which may have a “snowflake” chondroid matrix. However, completely lytic lesions are not unusual (Figure 14, DICOM images available: 14A, 14B, 14C, and 14D, courtesy of Claron Technologies). Cortical erosion, soft-tissue extension, and pain may or may not be present. Low-grade chondrosarcoma can be difficult, if not impossible, to differentiate from an enchondroma, which is the primary alternative consideration in the differential diagnosis. On MRI, low grade, differentiated chondrosarcomas will appear bright on T2 imaging, with more homogeneous signal than that of higher-grade lesions.3,4
Undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma) and fibrosarcoma
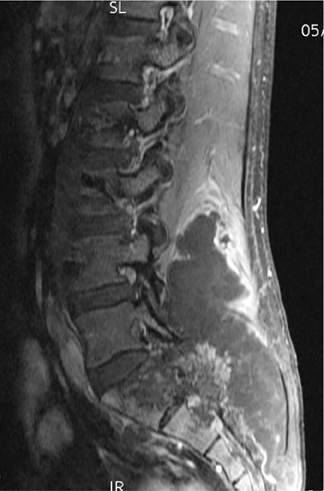
Although these two lesions have differing histologic appearances, they are indistinguishable radiographically. These are relatively rare bone tumors that may arise de novo or secondary to an underlying osseous lesion, such as Paget’s disease, fibrous dysplasia, or nonossifying fibroma, or at sites of prior radiation. This lesion has a permeative appearance with an indistinct ZOT and may or may not have an associated soft tissue component (Figure 15). The MRI appearance is again nonspecific, with low T1 signal and heterogeneously bright signal on T2-weighted images.3,7
Metastatic disease and myeloma
Osseous metastatic lesions have multiple appearances and therefore are often included in the differential for atypically appearing sclerotic or lytic lesions. Several metastatic lesions, such as renal cell, thyroid, melanoma, and choriocarcinoma, are classically lytic in appearance. However, other than renal cell cancer, this need not always be the case. Metastatic prostate cancer (Figure 16) will typically show sclerotic lesions, but breast, lung (Figure 17) and gastrointestinal malignancies can also mimic this appearance. As is the case with other lesions, MRI is useful for evaluation of soft tissue extent and staging; however, biopsy is needed for diagnosis.3-5
Multiple myeloma is a diffuse, permeative malignancy found in adults over the age of 35 to 40 yrs. While this lesion is typically lytic, a rare form of sclerotic myeloma is seen in association with POEMS syndrome (Polyneuropathy, Organomegally, Endocrinopathy, Monoclonal gammopathy and Skin changes). In the early stages, myeloma may be preceded by a predominantly lytic plasmacytoma that is usually found in the axial skeleton. Multiple myeloma ultimately ensues, typically with a diffuse axial and appendicular distribution. Multiple “punched out” lesions in the calvarium and axial skeleton are a classic appearance of this disease (Figure 6). Patients inevitably suffer from renal failure secondary to Bence-Jones protein deposition or associated amyloidosis. Of note, radionuclide studies with Tc99m-MDP are ineffective in demonstrating myelomatous lesions.3,4
Conclusion
This article has reviewed the commonest benign and malignant bone tumors the radiologist is likely to encounter. To construct a meaningful differential diagnosis, an attempt should be made to characterize the lesion as having either an aggressive or a benign appearance by assessing such qualities as the zone of transition, periostitis, pattern of lysis, and location. A careful and methodical analysis will help the radiologist determine if the lesion is likely benign or if additional workup or biopsy should be considered.
References
- Resnick D. Tumors and Tumor-Like Lesions of Bone: Radiographic Principles. In: Resnick D. Bone and Joint Imaging 2nd Ed. Philadelphia, PA: WB Saunders; 1996:979-990.
- Miller TT. Bone tumors and tumor-like conditions: Analysis with conventional radiography. Radiology. 2008;246:662-674.
- Manaster BJ. Tumors. In: Manaster BJ, Disler DG, May DA, Sartoris D. Musculoskeletal Imaging: The Requisites. 2nd Ed. St Louis, MO: Mosby; 2002:1-104.
- Helms CA. Malignant Bone and Soft Tissue Tumors. In: Brant WE, Helms CA. Fundamentals of Diagnostic Radiology. 3rd Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1086-1101.
- Resnick D. Skeletal Metastasis. In: Resnick D. Bone and Joint Imaging 2nd Ed. Philadelphia, PA: WB Saunders; 1996:1076-1091.
- Helms CA. Benign Cystic Bone Lesions. In: Brant WE, Helms CA. Fundamentals of Diagnostic Radiology. 3rd Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1063-1085.
- Resnick D, Greenway GD. Tumors and Tumor-Like Lesions of Bone: Imaging and Pathology of Specific Lesions. In: Resnick D. Bone and Joint Imaging 2nd Ed. Philadelphia, PA: WB Saunders; 1996:991-1063.
- Levine SM, Lambiase RE, Petchprapa CN. Cortical lesions of the tibia: Characteristic appearances at conventional radiography. Radiographics. 2003;23:157–177.
- Murphey MD, Andrews CL, Flemming DJ, et al. Primary tumors of the spine: Radiologic-pathologic correlation. Radiographics. 1996;16:1131-1158.
- Feldman F. Tuberous Sclerosis, Neurofibromatosis and Fibrous Dysplasia. In: Resnick D. Bone and Joint Imaging 2nd Ed. Philadelphia, PA: WB Saunders; 1996:1196-1210.
- James SLJ, Davies AM. Giant-cell tumours of bone of the hand and wrist: A review of imaging findings and differential diagnoses. Eur Radiol. 2005;15:1855–1866.
- Rodallec MH, Feydy A, Larousserie F, et al. Diagnostic imaging of solitary tumors of the spine: What to do and say. Radiographics. 2008;28:1019-1041.
- Murphey MD, Flemming DJ, Boyea SR, et al. Enchondroma versus chondrosarcoma in the appendicular skeleton: Differentiating features. Radiographics. 1998;18:1213–1237.
- Kitsoulis P, Galani V, Stefanaki K, et al. Osteochondromas: Review of the clinical, radiological and pathological features. In Vivo. 2008;22:633-646.
- Murphey MD, Choi JJ, Kransdorf MJ, et al. Imaging of osteochondroma: Variants and complications with radiologic-pathologic correlation. Radiographics. 2000;20:1407-1434.
Related Articles
Citation
Bone tumors and tumor-like conditions of bone. Appl Radiol.
September 28, 2012