AI-enabled CT, MR solutions and Helium-Free Mobile MR Are Key Highlights for Philips at ECR 2024
Images
Debuting in the Philips booth at ECR 2024 is the new AI-enabled CT 5300 system and the first BlueSeal MR mobile unit developed in Europe for Denmark-based Agito Medical Imaging. The company also announced it is teaming up with Synthetic MR for AI-based quantitative brain MR imaging.
Philips CT 5300 meets advanced diagnostic imaging requirements for cardiac care patient guidelines, as well as for other challenging areas such as trauma care and interventional procedures. The system also integrates virtual tools for real-time collaboration and clinical/technical support, which can be instrumental in overcoming challenges related to increased patient caseloads, complex cases, staff shortages, and budget constraints. Emphasizing lifetime value and sustainability, the CT 5300 is designed to lower energy consumption.
Regarding attendees’ response at ECR 2024 to the new CT 5300 system, Mark Olszewski, CT Product Management Marketing Leader at Philips says, “We are getting phenomenal feedback, whether it’s from the clinical insights that they're seeing with the cardiac performance that the system has, whether it's the automation and the AI workflow enhancements that it has to help departments deal with their higher workloads and their staff shortages, or whether it's really how the total cost of ownership comes together for the system over its lifetime. There's something in it for everybody.”
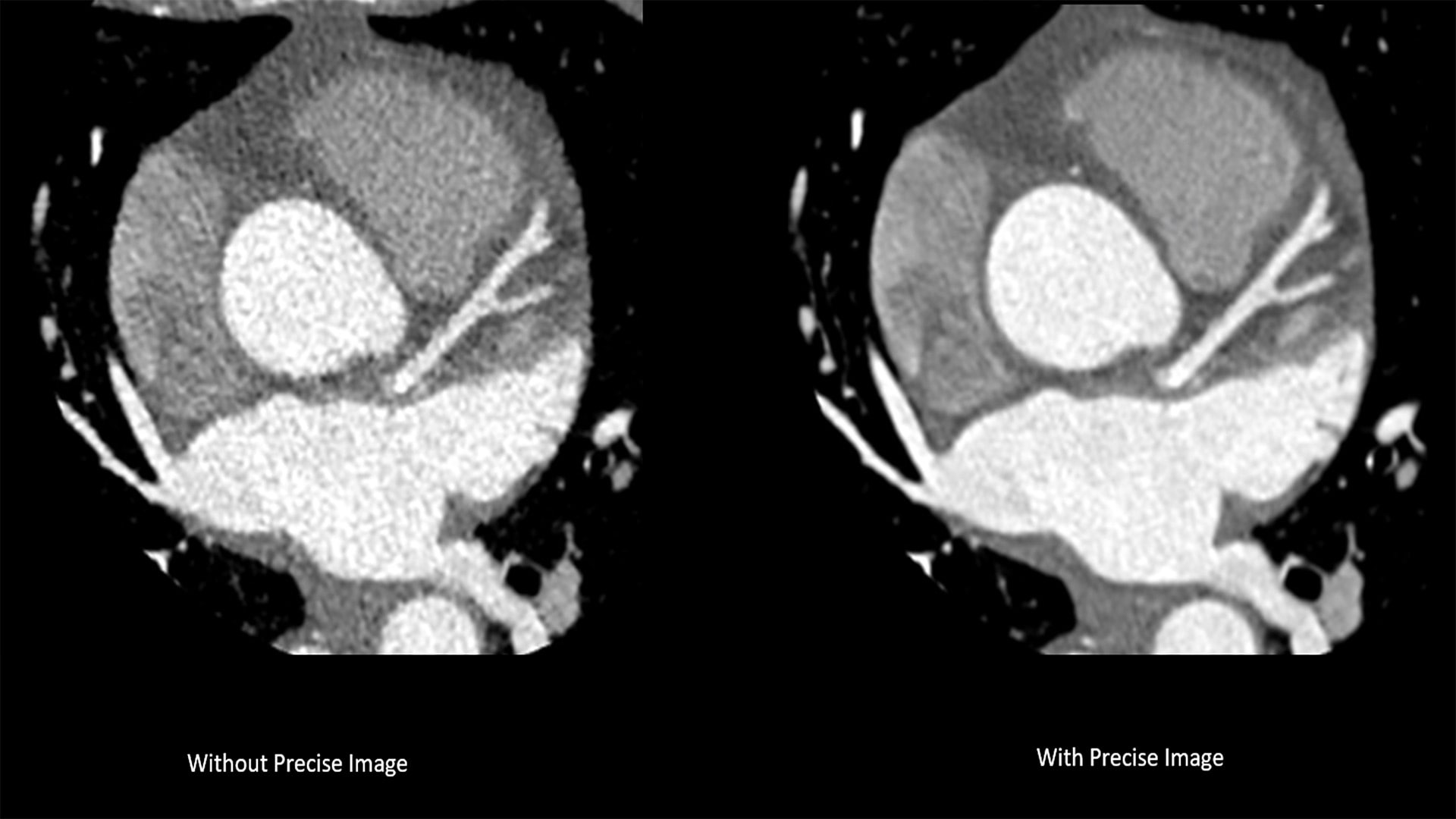
The new system introduces Nanopanel Precise, the industry’s first detector built from the ground up specifically for AI-based reconstruction. This brand-new detector leverages the full capabilities of Philips Precise Image reconstruction software to deliver high-quality images at much lower radiation dose. At 80% lower dose, Precise Image achieves up to 85% lower noise and 60% better low-contrast detectability than conventional image reconstruction. Combined with Precise Cardiac motion compensation, Precise Image makes CT 5300 particularly suitable for high-quality, motion-free, cardiac imaging in patients with high heart rates or heart-rate variability.
“We are really looking at how we are using AI thoughtfully all the way across how our customers use our system or (how they) need our system to perform,” says Danielle McCann, CT Content Strategist at Philips.
In addition to announcing the that more than 1,000 Philips BlueSeal 1.5T magnets are now installed worldwide, the company highlighted its first BlueSeal MR mobile unit designed in Europe. With a magnet that is 900 kg lighter than a traditional system and doesn’t require a quench pipe – required for traditional MR systems to safely expel their large volume of helium out of a building quickly in case of an emergency – Philips MR systems equipped with BlueSeal magnets can be installed in places that were previously unthinkable.
McCann notes that the helium-free mobile BlueSeal mobile MR unit is a continuation of the company’s commitment to providing this technology since its introduction in 2018, having saved more than 1.9 million liters of helium in the last six years.
“With the scarcity of helium and with very challenging climates and terrains in certain parts of the world, like Japan and Indonesia, this is going to allow the truck to be brought into areas where the (MR) technology would never be accessible before,” McCann says.
By dramatically easing installation requirements and addressing helium scarcity, both the Philips BlueSeal 1.5T magnet and the BlueSeal mobile MR unit are helping more patients in diverse locations benefit from a technology that plays an essential role in diagnosing many of the world’s most prevalent diseases.
“The BlueSeal Mobile unit shows just how you can bring access to care to more people by not having the barriers to entry of having the need for helium or the need for quench pipes,” Olszewski adds.
Philips also announced a collaboration with Synthetic MR to launch Smart Quant Neuro 3D, an objective decision support solution for diagnosis and therapy assessment of brain disorders like multiple sclerosis (MS), traumatic brain injury (TBI), and dementia.
Smart Quant Neuro 3D combines Philips’ AI based SmartSpeed image-reconstruction technology, Philips 3D SyntAc clinical application and Synthetic MR’s SyMRI NEURO 3D quantitative tissue assessment software. The combined offering gives healthcare providers powerful tools for greater diagnostic confidence ultimately benefiting patients. Philips’ exclusive agreement with SyntheticMR makes it the only company currently able to offer SyMRI NEURO 3D capability on MR scanners.
Smart Quant Neuro 3D leverages the power of AI to provide fully verified automatic and precise 3D segmentation and volume measurement of brain tissue such as white matter, gray matter, cerebrospinal fluid, and myelin. When interpreted by a trained physician, these measurements can provide useful information in determining diagnosis for patients.
“Life-changing brain injury and neurodegenerative disease are two of the most difficult diagnoses that clinicians have to make on a daily basis, because of the different symptoms exhibited by individual patients,” said Ruud Zwerink, Business Leader of MR at Philips. With Smart Quant Neuro 3D, clinicians have access to an easy-to-use tool to provide valuable quantitative data to track the impact of treatments and make informed decisions about adjustments or alternative interventions as needed. This continuous monitoring enhances patient care by enabling proactive management of neurological conditions and optimizing therapeutic outcomes for patients.
According to Philips, Smart Quant Neuro 3D is particularly effective at measuring myelin, the insulating layer around nerves that ensures electrical impulses in the brain reach their destination. Loss of myelin is a major characteristic of TBI, where Smart Quant Neuro 3D’s ability to detect abnormalities that today remain invisible on conventional MR scans, helps with early diagnosis and intervention. By quantitatively measuring myelin, clinicians can better understand the extent and impact of TBI and differentiate TBI from other neurological diseases.