A five-step strategy for building an LDCT lung cancer screening program
Images





As of October 2017, Baptist Health South Florida had screened more than 2400 patients at high risk for lung cancer with low-dose CT (LDCT). Here we will present our initial experience developing an LDCT lung cancer screening program, and our strategy for ensuring its success. This strategy included organizing a multidisciplinary team, defining local opportunities, developing standardized workflows, launching a marketing campaign, and implementing quality improvement (Figure 1). We believe this article can serve as a useful guide for other community health systems aiming to establish their own lung cancer screening programs.
Baptist Health South Florida is a non-profit community health system, spanning Miami-Dade, Broward and Monroe counties in southeastern Florida. We initiated a lung cancer screening program in November 2013, and subsequently became an American College of Radiology (ACR) Lung Cancer Center of Excellence.
Developing a successful lung cancer screening program within a large community health system such as ours presented unique challenges that had not been extensively reviewed at the time of this writing. Implementation of lung cancer screening throughout the country depends on data relevant to individual practitioners and health systems. Therefore, there is a need for community-driven data on CT lung cancer screening.
About lung cancer and screening programs
As the leading cause of cancer death in the United States (221,200 new cases and 158,040 deaths estimated for 2015),1 lung cancer is an attractive target for screening. Results from the landmark National Lung Screening Trial (NLST)2, 3 in 2012 demonstrated a 20% reduction in lung cancer-specific mortality among CT-screened participants compared to participants screened with chest radiography.
The mortality benefit of CT screening was likely underestimated, as subjects in the NLST were followed for only a short time. In December 2013, the United States Preventative Services Task Force (USPSTF) issued a grade B recommendation for LDCT lung cancer screening,4 which preceded a positive coverage decision by the Centers for Medicare and Medicaid Services (CMS) in February 2015.5 Consequently, millions of Americans at high risk for lung cancer are now eligible for LDCT lung screening without insurance co-payment.
Since the release of the NLST results, guidelines and best practices for responsible lung cancer screening were conceived by a team of health professionals at the Lung Cancer Alliance, which evolved into the National Framework for Excellence in Lung Cancer Screening and Continuum of Care. To complement this effort and ensure high-quality standards for LDCT lung cancer screening and reporting, the ACR developed the Lung Imaging Reporting and Data System (Lung-RADS), a structured, decision-oriented reporting system analogous to the BI-RADS system widely used in breast cancer screening.6 Lung-RADS is intended to link pulmonary nodule management pathways to the variety of nodules encountered on LDCT screening exams, and to minimize the rate of false-positive studies.
Recognizing the potential impact for LDCT screening to lower lung cancer mortality, the ACR later introduced the Lung Cancer Screening Center designation, built upon the ACR CT accreditation program.7 For a facility to be granted CT accreditation, it must receive a passing score in several areas of evaluation, including personnel qualifications, quality control/quality assurance, and image quality.8
The number of hospital systems and health care networks offering CT lung cancer screening has dramatically increased over the past several years. As demand for lung cancer screening increases, there is a need to validate the performance and feasibility of LDCT screening in clinical practice. McKee et al6 recently reported on the performance of ACR Lung-RADS applied to the lung cancer screening program at Lahey Clinic, an academic hospital in Burlington, MA. Between January 2012 and May 2014, more than 2000 high-risk patients were screened at Lahey Clinic with LDCT, and the application of ACR Lung-RADS increased the positive predictive value of screening by a factor of 2.5 compared to using positive thresholds from NLST. In a separate review, Lanni et al9 reported early results from the implementation of an LDCT lung cancer screening program at the Beaumont Health System, a large academic medical center in Royal Oak, MI.
Baptist Health’s strategy for building a screening program
Organize a multidisciplinary team
The initial step toward establishing an effective lung cancer screening program is assembling a strong multidisciplinary planning team. At Baptist Health South Florida (BHSF), our planning team included physician stakeholders from radiology, pulmonary medicine, cardiothoracic surgery, and primary care. Two part-time-equivalent nurses were included to serve as “patient navigators,” an essential link among patients, referring providers, and clinicians with expertise in lung nodule management. Other key personnel on the planning team included a lead CT technologist and hospital administrators. A well-respected physician champion was appointed from radiology to lead the planning team and serve as Program Director. The Program Director should satisfy ACR criteria for Lung Cancer Screening Center designation, including interpretation of at least 200 chest CT exams during the prior 36 months.7
Define local opportunities and challenges
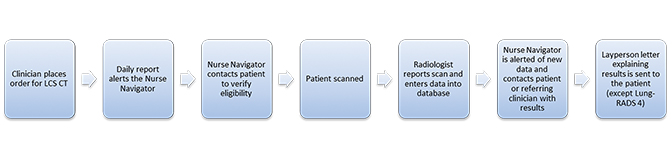
Once the planning team has been assembled, the Program Director should establish a clear vision for the program and set a realistic timeline for implementation. In preparation for the establishment of a new Miami Cancer Institute at BHSF, organ-specific disease management teams were created. The thoracic team was tasked with developing a CT lung screening program as part of a comprehensive approach toward identifying individuals at high risk for cancer. As the largest health system in Miami-Dade County, BHSF was uniquely positioned to provide broad geographic reach and a comprehensive diagnostic and treatment plan, including fellowship-trained chest and interventional radiologists, pulmonologists, and thoracic surgeons. A nurse specialist was also engaged to become the thoracic nurse navigator for the program. A flowchart should be developed that incorporates the process of order placement, patient screening, CT screening, and communication follow up. The flowchart should account for existing local policies and procedures to ensure smooth implementation. A sample flowchart from BHSF is included in Figure 2.
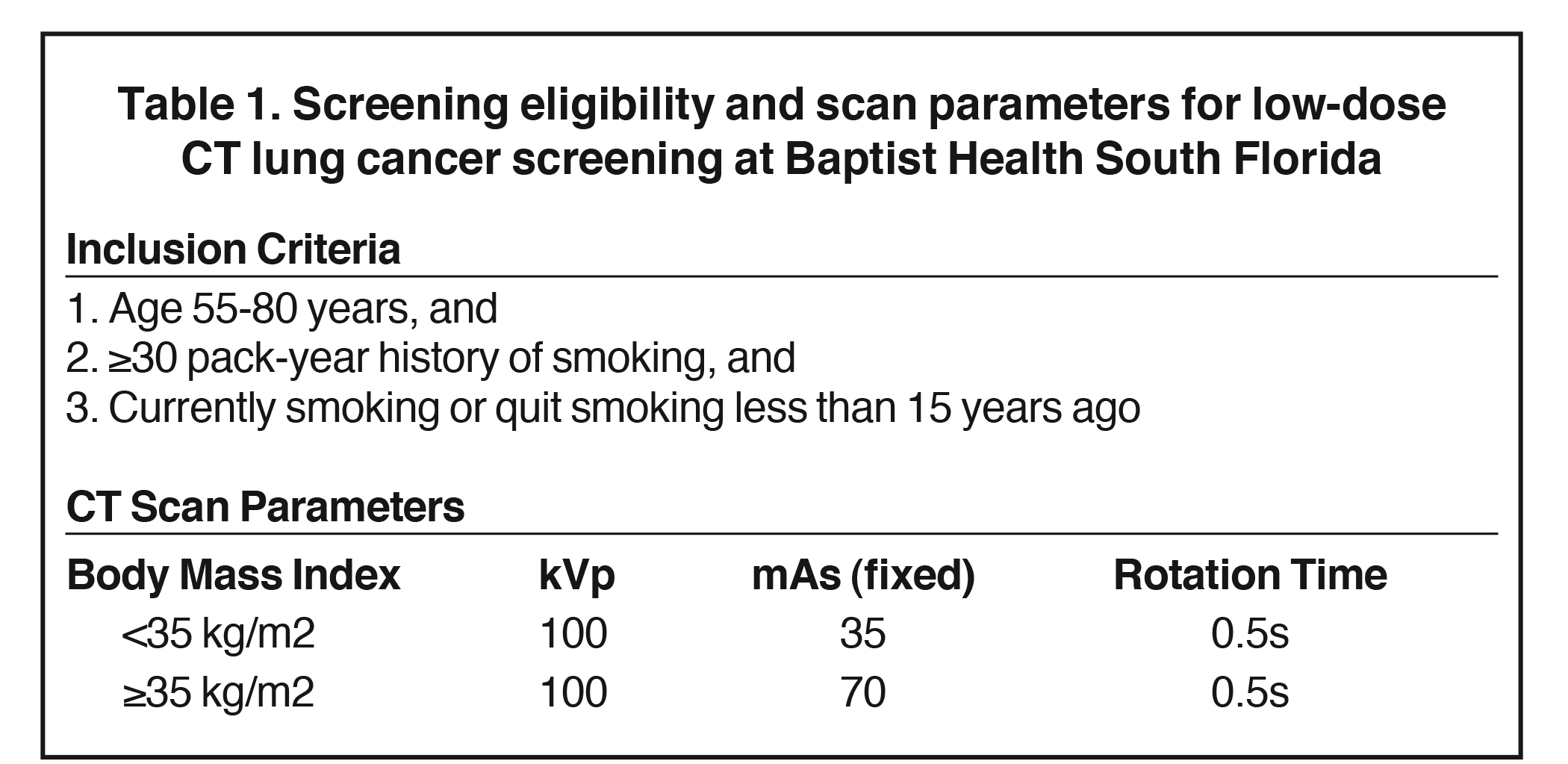
Eligibility criteria for annual LDCT lung screening covered by CMS include asymptomatic individuals aged 55-77 years with a high-risk tobacco smoking history, defined as at least 30 pack-years who are currently smoking or who had quit within the past 15 years. While age 77 is the maximum age set by CMS, the USPSTF recommended an upper age limit of 80 years for screening; this was the limit adopted by BHSF (Table 1).5
As the program was initiated in 2014, before insurance reimbursement for CT screening was widespread, the self-pay cost for the CT examination was set at $35. Practice parameters and technical standards for performance of LDCT lung cancer screening should be reviewed by the planning team to ensure all requirements are satisfied by the program at all of its imaging sites. CMS mandates a maximum dose threshold for CT lung screening defined by a volumetric CT dose index (CTDIvol) of 3 mGy for a standard-sized patient (5 feet 7 inches, 155 pounds) , with appropriate dose reduction for smaller patients and appropriate dose increase for larger patients. At BHSF, mAs is adjusted by body mass index (BMI), with 35 mAs used for smaller patients (BMI < 35 kg/m2) and 70 mAs used for larger patients (BMI ≥35 kg/m2).
Develop a standardized workflow
Establishing a standardized workflow for lung cancer screening is essential for the program to function smoothly. This becomes especially important to maintain consistency if CT imaging will be acquired across multiple imaging sites. Appropriate patient selection criteria must be rigidly followed to ensure reimbursement for the screening CT exams. A flowchart of the BHSF workflow is depicted in Figure 2. Standardized intake forms are generated by a nurse navigator who contacts the patient prior to the CT appointment to verify eligibility. Currently, BHSF does not accept self-referrals for lung cancer screening, and all patients are required to have a physician referral to ensure clinical follow up of abnormal results. Appointment reminders are generated by phone and regular mail to minimize the number of no-shows.
Only physicians credentialed in diagnostic radiology and radiation safety can review LDCT lung cancer screening exams and claim reimbursement. Per CMS guidelines, interpreting physicians must be board certified or board eligible by the American Board of Radiology or an equivalent organization. Further, interpreting physicians must have at least 300 documented chest CT cases over the preceding 3 years and must actively participate in continuing medical education (CME).
Structured reporting for lung cancer screening is essential to promote clear communication of results to guide appropriate patient management. The Lung-RADS lexicon should be utilized in the radiology report, including a final Lung-RADS assessment category and management recommendation. The structured reporting template used at BHSF is depicted in Figure 3. Descriptors for nodule location, morphology (solid, part-solid, ground-glass), size, attenuation (soft tissue, fat, calcification), and margins (spiculated, lobulated, smooth) should be included in the Findings section. At BHSF, additional qualitative assessment of coronary artery calcification (severe, moderate, mild, none) is reported for low-dose screening CTs to facilitate primary prevention of coronary artery disease, as recommended by ACR, Society of Cardiovascular Computed Tomography (SCCT) and the Society of Thoracic Radiologists (STR).10 There is considerable risk factor overlap for lung cancer and atherosclerotic heart disease. Coronary artery calcification can be readily observed on non-gated chest CT and correlates with adverse cardiovascular events.11
Launch a marketing campaign
Strategic marketing of a lung cancer screening program is essential for the program to grow and succeed. Successful practices recognize the value of marketing in targeting for both patients and referring physicians. Most patients are unaware that lung cancer screening programs exist, or that most insurance providers will cover CT screening with no out-of-pocket cost. Opportunities for effective, low-cost marketing include presentations at grand rounds, patient educational seminars, and in-person visits with local physician practices. Online marketing through social media and other platforms have become increasingly important for medical practice growth, as more patients and referring physicians seek healthcare resources on the web.12 Online marketing opportunities include search engine optimized blogs, social media (including Facebook, Twitter, LinkedIn), and targeted emails. Other marketing strategies such as newspaper, radio and television advertisements can also be highly effective, but at a higher cost. At BHSF, we sparingly used advertisements on public radio, but largely we leveraged in-system resources. Advertisements went into Baptist-produced patient-oriented magazines and marketing collateral; patient-facing seminars on smoking cessation and lung screening were offered at Baptist facilities; word-of-mouth education from radiologists to referring physicians was also important.
Referring physicians within the BHSF network represent the most common source of patient exposure to our lung cancer screening program (Figure 4). However, approximately one-third of patients were exposed to our program through non-physician sources, including newspaper/magazine advertisements (13%), internet/social media (9%), radio commercials (5%) and word of mouth (3%). For 36% of patients, the CT Lung Screening exam was the patient’s first imaging study at BHSF—this statistic suggests the CT lung screening program was successful in bringing new patients to Baptist Health.
Implement and iterate with quality improvement
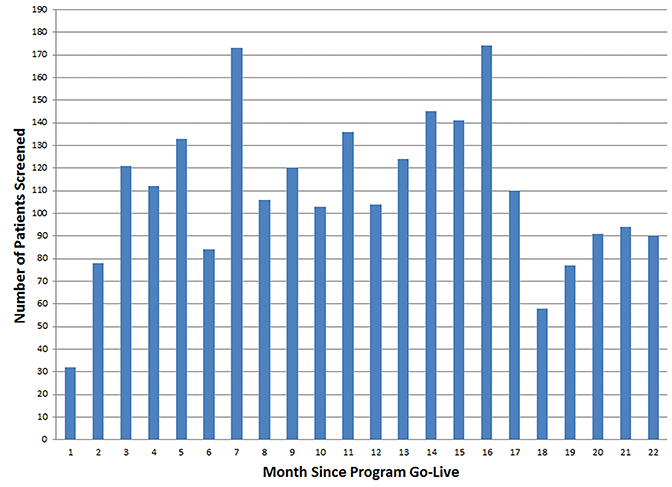
Following program implementation, volume trends for the screening program should be periodically reviewed to identify changes in referral patterns (Figure 5). We also recommend periodic evaluation of patient demographics and aggregate CT screening results, including a breakdown of total Lung-RADS assessment categories. This is helpful for characterizing the referral population and for tailoring the program over time to meet their specific needs.
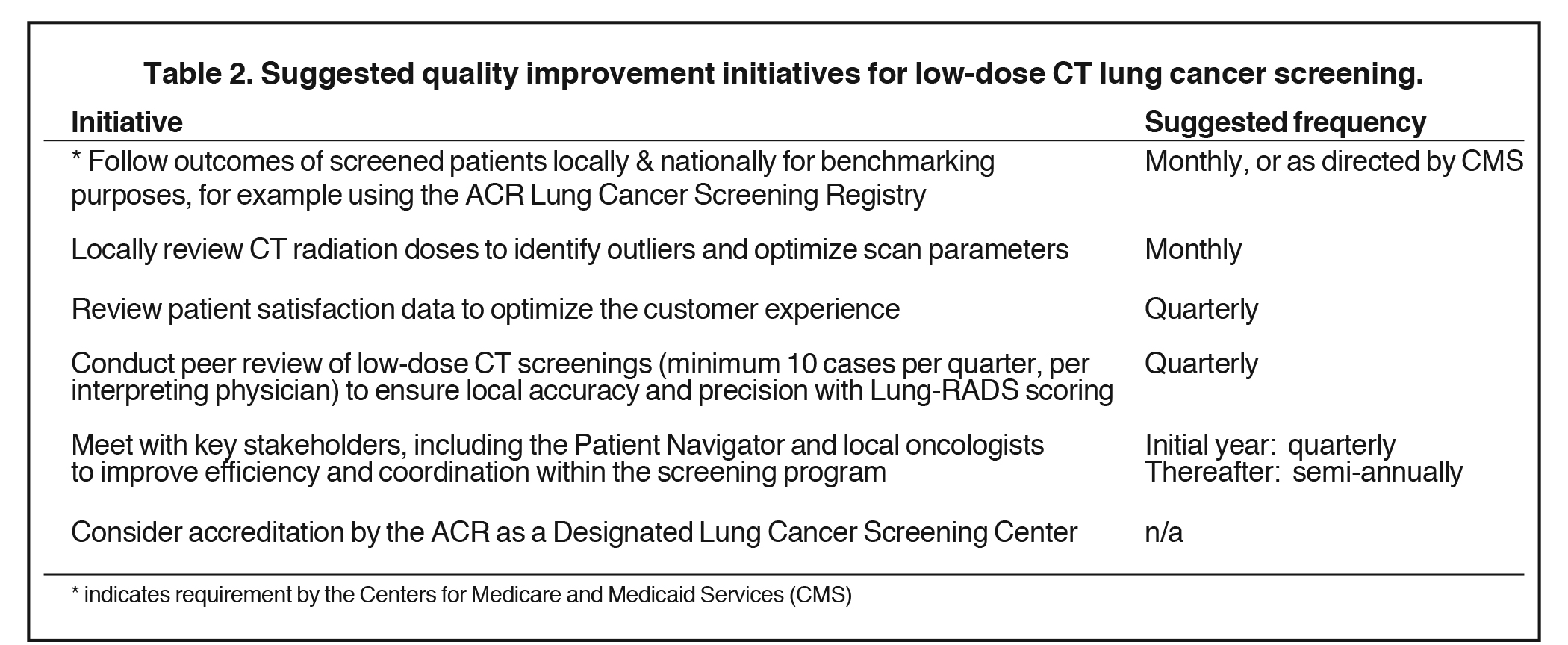
Quality improvement should be implemented for the lung cancer screening program to facilitate continuous improvements in clinical and diagnostic performance, as well as improving patient outcomes. Quality improvement should be a continuous process, ensuring the screening program remains competitive in a rapidly changing healthcare environment. Defining specific areas for quality improvement can be facilitated by patient and referring physician satisfaction surveys, identifying process bottlenecks, and performing regular walk-throughs. Specific recommendations are provided in Table 2. A multidisciplinary team approach is encouraged to address quality improvement initiatives, and the team should convene on a regular basis. An excellent general resource for quality improvement in radiology practice is provided by Krustal et al.13
Positive CT screening cases (Lung-RADS 3 and 4) should be followed to evaluate positive predictive value with respect to clinically diagnosed lung cancer and biopsy-proven lung cancer. In light of the CMS requirement to track outcomes of screened patients for national benchmarking, we recommend participating in the ACR Lung Cancer Screening Registry, which has already been approved by CMS. As BHSF physicians are spread over a large geographic area, we developed a virtual multidisciplinary committee, which discusses anonymized cases over encrypted email and phone—the radiologist initiates discussion with a brief history and an image of a suspicious Lung-RADS 4 lesion, and the pulmonologist, interventional radiologist, and thoracic surgeon contribute their thoughts on the likelihood of malignancy and the best approach for diagnosis. Reaching common ground among committee members is critical for ensuring the most appropriate management strategy is selected.
Results of a high-volume community screening program
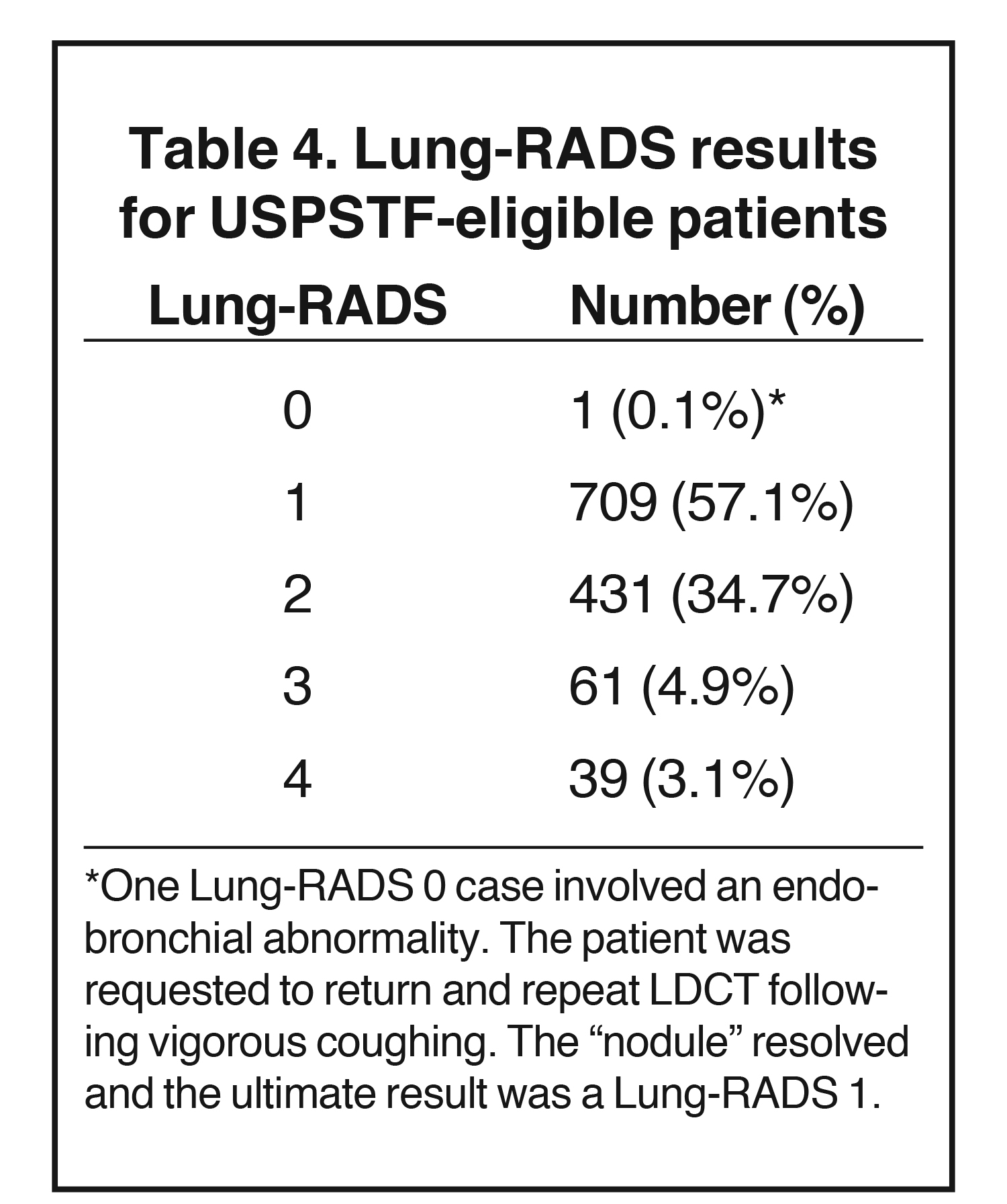
Baptist Health South Florida initiated its lung cancer screening program in July 2014 under the auspices of the Miami Cancer Institute (MCI) of Baptist Health. The thoracic tumor management team comprised a multidisciplinary group of physicians, administrators, and allied health professionals. One of this article’s authors (JCB) was the lead for radiology and created the CT lung screening program. Enrollment grew and reached a steady state of 5-7 LDCT examinations per weekday, quickly becoming one of the highest-volume programs in the nation. Data from the first 22 months (664 days) of the lung screening program were collected under IRB waiver (July 2014 - April 2016). Of 2403 screened patients, only USPSTF-eligible patients were included (n=1241). Baseline patient characteristics are included in Table 3. Of note, the lung screening CT was the first imaging study at BHSF for 36% of patients.
Most of the patients fell into ACR Lung-RADS category 1 (57.1%), and the vast majority fell into either Lung-RADS category 1 or 2 (total 91.9%). For all of these patients, the follow-up recommendation consisted of an annual routine low-dose chest CT as long as USPSTF-eligibility was maintained the following year. Lung-RADS results are summarized in Table 4.
Several additional parameters were captured at the time of LDCT. Radiation dose was quantified for each examination [assumed conversion factor of 0.014 mSv/(mGy·cm)]; the average radiation dose was 0.54 milliSieverts. In addition to the Lung-RADS score, each CT report also included data on the following: presence and severity of coronary artery calcium burden, presence or absence of interstitial lung disease, presence or absence of emphysema, presence or absence of vertebral body compression deformity, and presence or absence of an incidental significant finding. Coronary calcium burden was qualitatively assessed by an experienced cardiothoracic radiologist with 6 years of CT calcium scoring experience (JCB) into no calcium burden, mild calcium burden (estimated Agatston score 1-99), moderate calcium burden (estimated Agatston score 100-399), or severe calcium burden (estimated Agatston score >399).
An incidental significant finding was a finding unrelated to lung cancer that required further follow up—an “actionable” finding, such as an indeterminate adrenal nodule or axillary lymphadenopathy. The prevalence of these findings is shown in Table 5.
There was a low prevalence of interstitial lung disease (2.4%) and spinal compression deformity (1.2%). There was high prevalence of emphysema (33%) and coronary artery calcification (68.2%). The prevalence of both emphysema and coronary artery calcification were likely underestimated, as small amounts of emphysema and coronary artery calcium were more difficult to identify on these non-ECG-gated LDCT examinations.
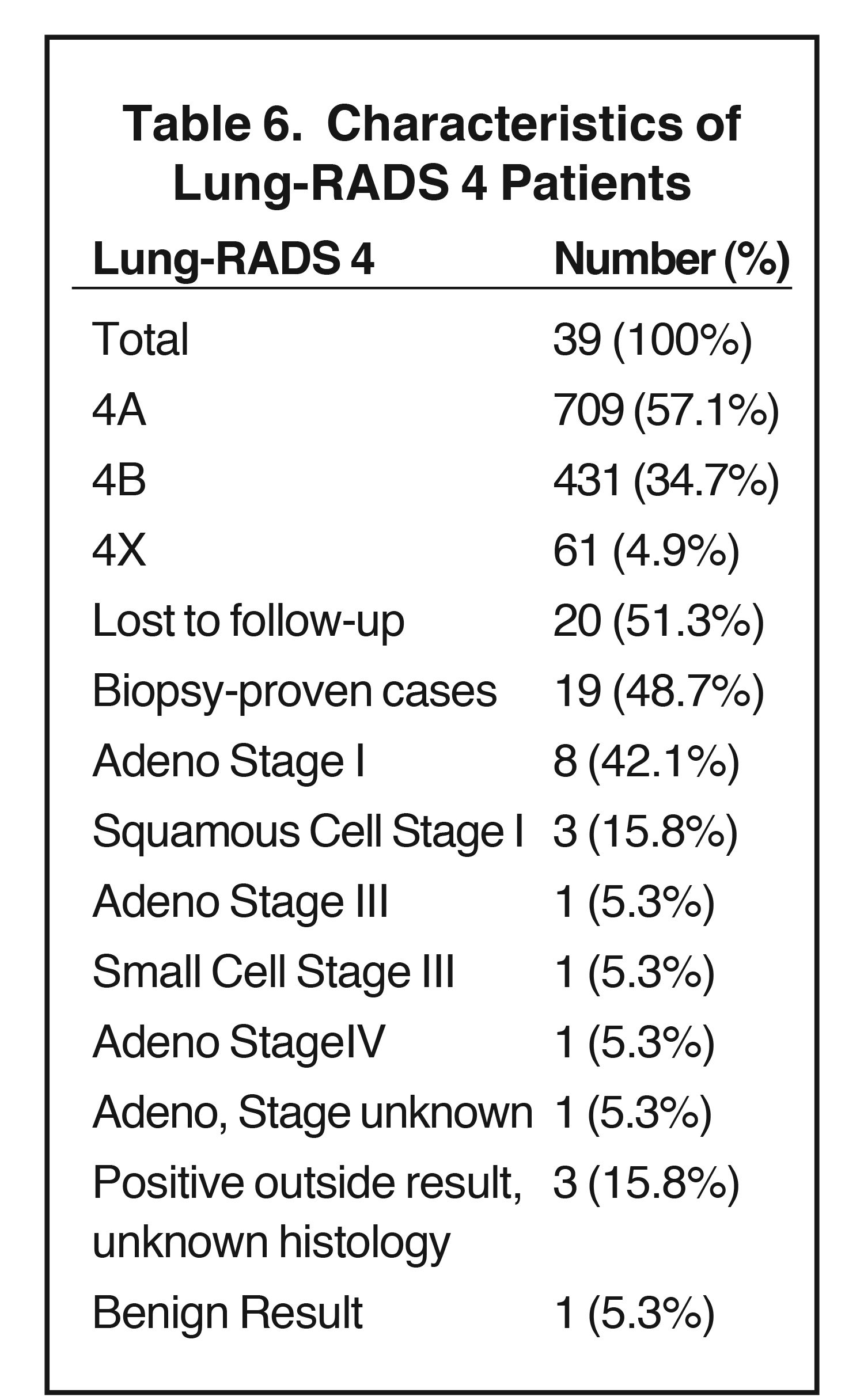
The total number of positive cases (Lung-RADS 3 or Lung-RADS 4) was 100, 8% of the total. This represents the “false positive ceiling,” as many of the Lung-RADS 4 cases were eventually determined to be true positives. No Lung-RADS 3 case was eventually proven to represent a malignancy; however, several of the Lung-RADS 3 cases involved persistent, large ,ground-glass nodules, which may represent indolent adenocarcinoma-in-situ. These cases, in the experience of the study team, are less likely to lead to biopsy and therefore suffer from continued uncertainty regarding histology. The Lung-RADS 4 cases are summarized in Table 6.
A large percentage of Lung-RADS 4 patients were lost to follow up (51.3%). BHSF hospitals are centered in Miami-Dade County, but Baptist Health outpatient centers performing LDCT exams span South Florida from northern Broward County to Monroe County to the south. Outpatient centers in Broward, in particular, have no nearby Baptist Health South Florida hospital for patients to undergo biopsy or surgery. Of the patients with known follow-up, the majority had early stage malignancy (58%). Only 1 patient had distant metastasis. Only 1 patient of the Lung-RADS 4 patients with follow-up had a benign result at pathology.
Limitations of our dataset include lack of itemized follow-up data on Lung-RADS 3 patients with regard to stability on subsequent CT scans, loss of follow up on approximately half of the Lung-RADS 4 patients, and lack of clinical follow up on Lung-RADS 1 and 2 patients to determine the false negative rate.
Conclusions
Since CMS approved coverage of LDCT for lung cancer screening in 2015, the number of lung cancer screening programs is expected to rise. As such, there will be demand for community health systems to develop effective screening programs.14 A successful CT lung cancer screening program requires a strong multidisciplinary team, defined local opportunities, standardized workflows, strategic marketing, and quality improvement.
This strategy has contributed to the success of lung cancer screening at BHSF. Lung cancer screening results for our high-volume community program demonstrated data concordant with known lung cancer screening literature, including a low rate of false positive examinations, low radiation dose with an average dose similar to that of 4-view screening mammography, a low rate of significant incidental findings, and a majority of cancer-proven patients having early-stage, treatable disease.
In particular, the community setting for this study illustrates that a tertiary or academic center is not required to ensure a low rate of false positives or significant incidental findings. For many patients, despite a minimum age of inclusion of 55, the LDCT exam was their first contact with BHSF radiology.
References
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer 2016. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed November 6, 2017.
- National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The national lung screening trial: Overview and study design. Radiology. 2011;258(1):243-253.
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
- Moyer VA. Screening for lung cancer: U.S. Preventive services task force recommendation statement. Ann Inter Med. 2014;160(5):330-338.
- Retrouvey M, Patel Z, Shaves S. US preventive services task force CT lung cancer screening recommendations: Community awareness and perceptions. J Am Coll Radiol. 2016;13(2 Suppl):R35-37.
- McKee BJ, Regis SM, McKee AB, et al. Performance of ACR lung-RADS in a clinical CT lung screening program. J Am Coll Radiol. 2016;13(2 Suppl):R25-29.
- Kazerooni EA, Armstrong MR, Amorosa JK, et al. ACR CT accreditation program and the lung cancer screening program designation. J Am Coll Radiol. 2016;13(2 Suppl):R30-34.
- Fintelmann FJ, Bernheim A, Digumarthy SR, et al. The 10 pillars of lung cancer screening: Rationale and logistics of a lung cancer screening program. Radiographics. 2015;35(7):1893-1908.
- Lanni TB, Jr., Stevens C, Farah M, et al. Early results from the implementation of a lung cancer screening program: The Beaumont Health System experience. American Journal of Clinical Oncology. 2015 (Dec 8). Doi: 10.1097/COC.0000000000000254
- Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017; 11(1):74-84.
- Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345.
- Levin DC, Rao VM, Flanders AE, et al. Marketing a radiology practice. J Am Coll Radiol. 2016;13(10):1260-1266.
- Kruskal JB, Eisenberg R, Sosna J, et al. Quality improvement in radiology: Basic principles and tools required to achieve success. RadioGraphics. 2011;31(6):1499-1509.
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: A systematic review. Jama. 2012;307(22):2418-2429.
Citation
JC B, CD M, MA L, RC C.A five-step strategy for building an LDCT lung cancer screening program. Appl Radiol. 2018; (1):12-18.
January 11, 2018