18F-FDG PET/MR exams don’t need gadolinium chelates for pediatric tumor evaluation
Administration of gadolinium (Gd) chelates is not necessary for accurate diagnostic characterization of most solid pediatric malignancies on18F-FDG PET/MR images, according to a study published online October 15, 2015 in the Journal of Nuclear Medicine. This finding could streamline scans for combined local and whole-body tumor staging, making the procedure more economical and practical when imaging pediatric patients.
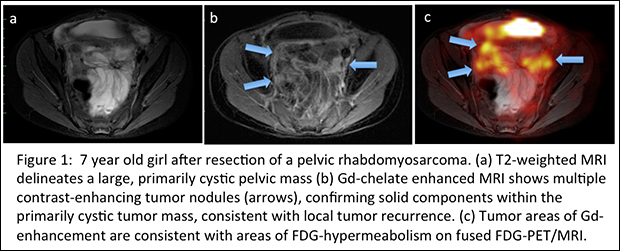 The study by a multidisciplinary team at the Stanford School of Medicine involved 119 patients from infancy to the age of 25 who had been diagnosed with a solid tumor mass and were referred to an MRI scan of the abdomen or pelvis. In a first step, the researchers compared the diagnostic accuracy of unenhanced T2-weighted fast-spin-echo images and unenhanced diffusion-weighted images with Gd-chelate enhanced T1-weighted 3D spoiled gradient echo images.
The study by a multidisciplinary team at the Stanford School of Medicine involved 119 patients from infancy to the age of 25 who had been diagnosed with a solid tumor mass and were referred to an MRI scan of the abdomen or pelvis. In a first step, the researchers compared the diagnostic accuracy of unenhanced T2-weighted fast-spin-echo images and unenhanced diffusion-weighted images with Gd-chelate enhanced T1-weighted 3D spoiled gradient echo images.
A subgroup of 36 patients had also undergone a18F-FDG PET study within three weeks of the MRI. The researchers fused the PET images with unenhanced T2-weighted MR images and with Gd-enhanced T1-weighted MR images and then compared the diagnostic accuracy of unenhanced18F-FDG PET/MRI and Gd-enhanced18F-FDG PET/MRI.
Three radiologists independently analyzed the MR images with respect to 14 diagnostic criteria, such as image quality, organ of origin, specific tumor characteristics, presence or absence of necrosis, types of metastases, infiltration of other organs, vessel delineation, extension across the midline, and tumor thrombosis. A pediatric radiologist and a nuclear medicine physician reviewed the18F-FDG PET/MR images regarding the 14 criteria and calculated diagnostic accuracies.
Principal investigator Heike E. Daldrup-Link, M.D., Ph.D., associate professor of radiology at Lucile Packard Children’s Hospital and the Molecular Imaging Program at Stanford, and colleagues reported that lesion-by-lesion analysis revealed concordance between18F-FDG avidity and gadolinium chelate enhancement for 106 of the 123 tumors. “Our data showed no significant difference in diagnostic accuracy between gadolinium chelate-enhanced and unenhanced MR images, or between gadolinium chelate-enhanced and unenhanced18F-FDG PET/MR images for staging of pediatric tumors,” they wrote. “We found diagnostic accuracy for local tumor staging to be similar between T2- or diffusion-weighted MR images and gadolinium chelate-enhanced MR images.” Because of this, they suggest that MR images for local staging can be streamlined and gadolinium chelates replaced by more specific MR contrast agents, with he possible exception of imaging focal liver lesions which diffusion-weighted and T2-weighted MR images have a limited ability to characterize.
Dr. Daldrup-Link told Applied Radiology that the results of this study convinced the pediatric oncology team at Stanford that Gd-chelates can be replaced by more specific MR contrast agents without the loss of clinically important information. This enabled the pediatric molecular imaging team at Stanford to initiate nanoparticle-enhanced18F-FDG PET/MRI scans for staging of pediatric tumors.
REFERENCE
- Klenk C, Gawande R, Tran VT, et al. Progressing Toward a Cohesive Pediatric18F-FDG PET/MR Protocol: Is Administration of Gadolinium Chelates Necessary? J Nucl Med. Published online October 15, 2015.