New Trends in the Diagnosis and Management of Renal AML Subtypes: Part II
Introduction
Up to 20% of all renal masses are benign, with the majority of these benign renal masses being angiomyolipomas (AMLs). The majority of AMLs contain macroscopic fat, which is their key imaging feature. Accurate diagnosis of these classic AMLs may help prevent unnecessary surgery or percutaneous ablation. These classic AMLs and their imaging features are discussed in more detail in Part I of this 2-part series.1
Most AMLs have a variable composition of smooth muscle, blood vessels, and fat, and this variability results in multiple subtypes. A minority of AMLs lack macroscopic fat, making differentiation from other renal masses, such as renal cell carcinomas (RCCs), much more challenging. This subset of AMLs has been described by various names and classified both by imaging and pathology, including fat-poor AML (fpAML), lipid-poor AML, AML with minimal fat, minimal-fat AML, and fat-invisible AML.2 In this review, we refer to this common, non-classic subtype as fpAMLs.
Because they lack the classic feature of macroscopic fat, fpAMLs have garnered significant attention in imaging literature. Most fpAMLs occur in female patients, and, while they tend to occur in patients at a younger age than RCCs, the age ranges can overlap.
There is no universally accepted classification system for fpAMLs.2 - 4 This may be due to their rarity compared to classic AMLs.
On CT, fpAMLs are classified as either hyperattenuating or isoattenuating. Other subtypes include rare variants such as AMLs with epithelial cysts, which are benign and contain a cystic component, and epithelioid variants, which may be malignant. Additionally, renal AMLs occur in association with tuberous sclerosis (TS) and, rarely, pulmonary lymphangioleiomyomatosis (LAM).2 - 4
Hyperattenuating fpAMLs
Most fpAMLs are hyperattenuating on CT (Hounsfield units >45) owing to the absence of macroscopic fat. This increased density distinguishes fpAMLs from many RCCs ( Figure 1 , Table 1 ). Without macroscopic fat, the muscular portion of the AML becomes the dominant component of the mass, leading to higher attenuation values. The attenuation of the mass can be compared with that of the adjacent renal cortex using the tumor-to-cortex ratio (TCR). In one study by Jeong, the TCR on unenhanced CT was 1.37 for fpAML and 0.83 for clear cell RCC (ccRCC).5 This higher density is helpful in distinguishing fpAML from most RCCs, particularly ccRCC, though the TCR for fpAML and chromophobe RCC is similar. Hyperattenuating fpAMLs rarely contain cysts or calcifications. In a series by Ma, calcifications were observed in 12.9% of RCCs compared with only 4.5% of fpAMLs, but this difference was not statistically significant. In contrast, cystic degeneration on CT was seen in 61.3% of ccRCCs compared with only 9.1% of fpAMLs, a difference that contributed to a radiomics-based CT model in distinguishing between these 2 masses.6
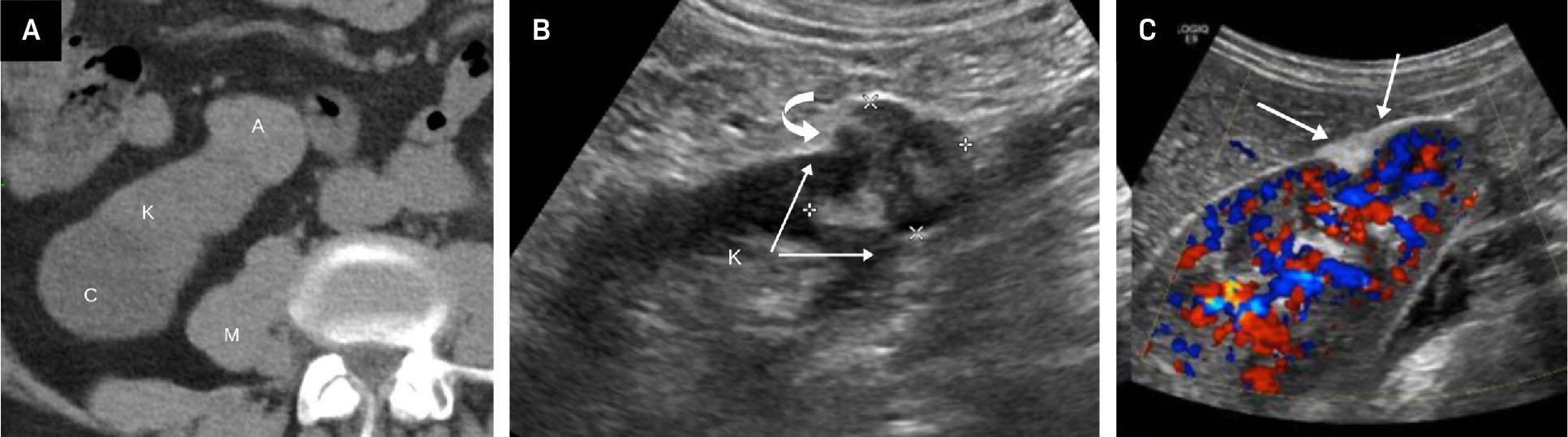
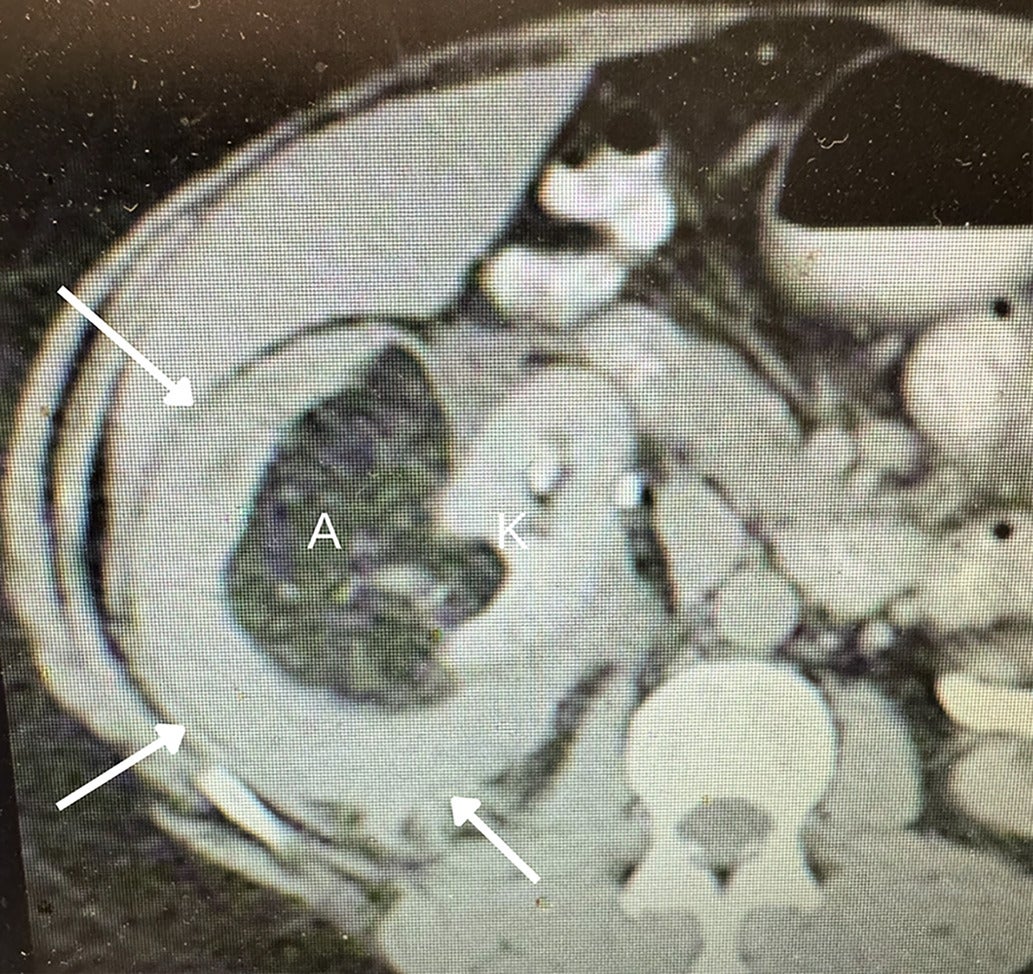
Fat-poor angiomyolipoma (fpAML) with angular interface. CT scan (A) showing the right kidney [K] with a simple cyst [C] and a fpAML [A]. The Hounsfield unit of the fpAML was 50 and identical to the HU of the adjacent muscle [M]. US showed this fpAML (B) to have mixed echogenicity, with an angular interface (arrows) and some overflow of the renal cortex (curved arrow, K = kidney). US of peripheral AMLs (C), which, like fpAMLs, often have the “overflowing beer” sign (arrows) with the renal cortex. The presence of either an angular interface or “overflowing beer” sign is helpful in distinguishing fpAMLs from renal cell carcinomas.
CT and MRI Features of Hyperattenuating Fat-Poor Angiomyolipomas
| CT | MRI |
|---|---|
| Hounsfield unit >45 with no fat attenuation | T2-weighted—homogeneously hypointense |
| Variable, frequently homogeneous, early enhancement | Fat sat—no signal loss |
| No fat attenuation | Chemical shift—usually no signal loss |
| No calcifications or cysts | No cysts |
On MRI, a hyperattenuating AML appears hypointense on T2-weighted images ( Figure 2 , Table 1 ), whereas ccRCCs are typically hyperintense, aiding their differentiation. However, papillary RCC (pRCC) can also appear hypointense on T2, potentially mimicking fpAML. As with density comparisons on CT, the T2 signal intensity (SI) of these masses on MRI can be quantified using the TCR, which compares the SI of the mass to that of the spleen or adjacent renal cortex. In a study by Jeong et al, the mean TCR for fpAML was 0.75, significantly lower than the 1.21 reported for ccRCC.5 Setting the TCR threshold at 0.86 achieved a sensitivity of 93% and a specificity of 82% for distinguishing fpAML from ccRCC.
Hyperattenuating fat-poor angiomyolipoma (fpAML) in an adult female. US of the right kidney showing an echogenic mass (arrow) (A). Non-contrast CT shows the mass to be hyperattenuating, with Hounsfield units >45 (arrow) (B). Axial single-shot fast spin echo (SSFSE) T2-weighted MRI shows the mass to be of low signal intensity (arrow) (C). Contrast-enhanced LAVA FLEX (FAT SAT) MRI shows early contrast enhancement of the mass (arrow) (D). Chemical shift imaging with in-phase LAVA FLEX coronal MRI showing the mass (arrow) (E). Chemical shift imaging with out-of-phase LAVA FLEX coronal MRI shows no signal drop-out within the mass (arrow) indicative of no microscopic fat (F).
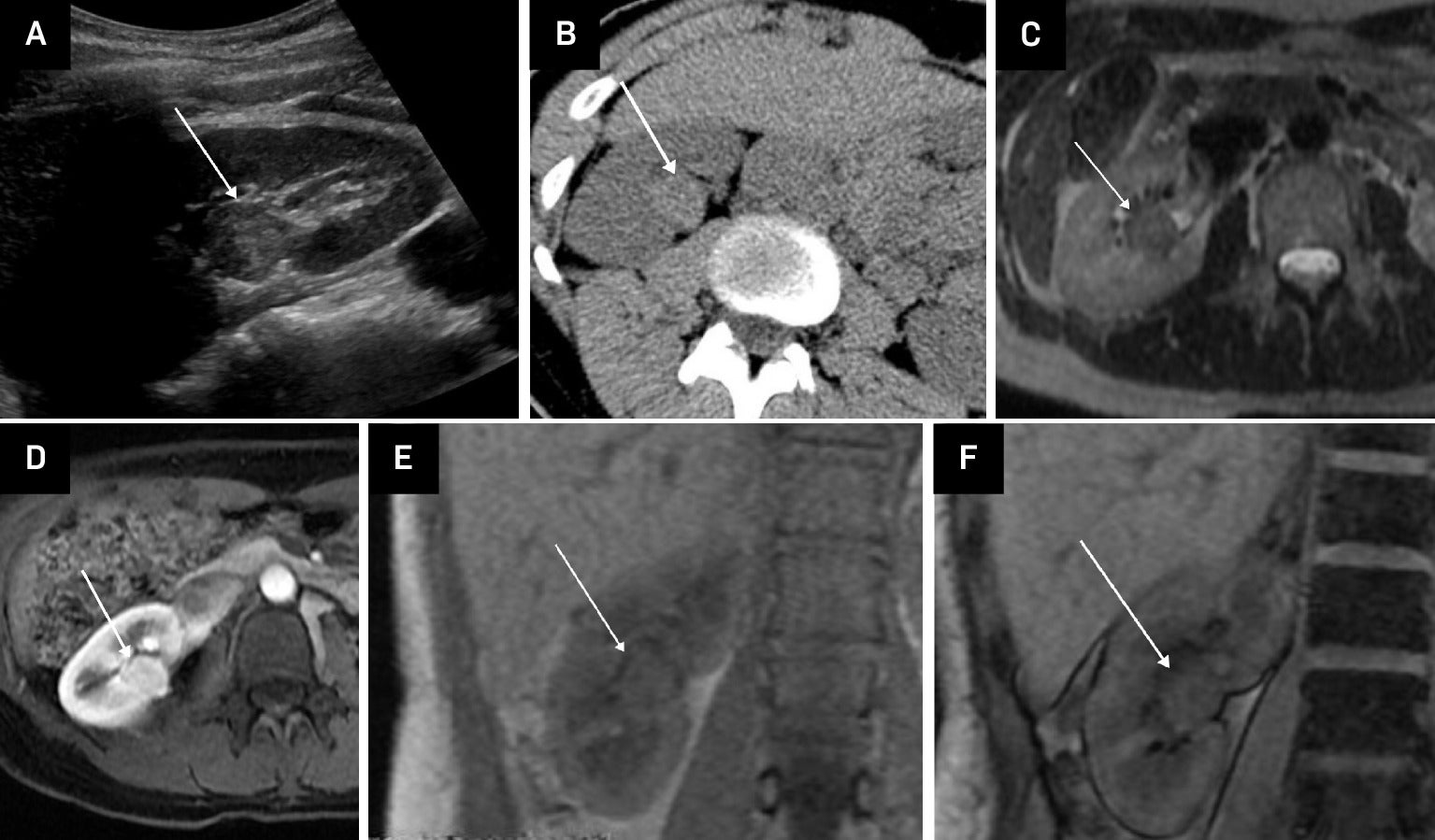
Chemical shift imaging may assist in distinguishing fpAMLs from pRCCs.5 Most fpAMLs demonstrate no signal loss on out-of-phase chemical shift images, although occasional small foci of signal drop may be present due to microscopic fat ( Figure 2 ). By comparison, ccRCCs possibly show diffuse signal loss on opposed-phase imaging due to the presence of intracytoplasmic lipid.3, 4
On contrast-enhanced imaging, fpAMLs are usually homogeneously avidly and rapidly enhancing, whereas pRCCs demonstrate delayed contrast enhancement.7 Arterial-to-delayed enhancement ratios on MRI have been proposed to differentiate fpAMLs from RCC subtypes. Using dynamic contrast-enhanced spoiled gradient-echo images, arterial, venous, and 3-minute-delayed phases can be obtained. A 5 mm or larger region of interest is placed in the most arterial enhancing portion of the mass as the SI. The ratio is calculated as follows: (ST arterial – SI pre)/(SI delayed – SI pre). An arterial-to-delayed enhancement ratio >1.5 for T2-hypointense lesions can aid differentiation between the fpAMLs and all subtypes of AMLs.8
Park et al examined US features of renal masses with low T2 SI on MRI and found that 45% of fpAMLs were hyperechoic, whereas none of the low T2 RCCs demonstrated hyperechogenicity.9 Thus, approximately half of fpAMLs exhibit the hyperechogenic appearance seen in classic AMLs.
As we discussed more extensively in Part I, morphological features help distinguish classic AML from RCC on US, and they can similarly aid in differentiation of fpAML.1 Kim et al found that on contrast-enhanced CT of renal masses <4 cm, an angular interface had a sensitivity of 55% and specificity of 81.9% for fpAMLs, while the “overflowing beer” (also referred to as the “drooping”) sign carries a sensitivity of 61% and specificity of 97.10 Strother et al demonstrated that both signs were strongly associated with fpAML, with odds ratios of 12.6 and 11.2, respectively ( Figure 1 ).11 Thus, when an angular interface or overflowing beer sign is present, the lesion is more likely an AML than RCC.
Isoattenuating fpAMLs
There are other subtypes of AMLs, including isoattenuating AMLs, AMLs with epithelial cysts, and epithelioid AMLs ( Table 2 ). Isoattenuating fpAMLs are rarer than hyperattenuating AMLs and have Hounsfield units between –10 and 45. Like the hyperattenuating fpAML, these types lack macroscopic fat, cysts, or calcifications. On MRI, isoattenuating fpAMLs are typically T2 hypointense and do not exhibit signal drop-out using fat saturation techniques, though signal drop-out is occasionally seen on out-of-phase chemical shift imaging. Due to overlapping features with RCC, differentiation can be challenging, and biopsy is often required.5 However, in select cases with more characteristic features, imaging surveillance may suffice.
Other Subtypes of Angiomyolipomas and Their Imaging Features
| CT | MRI | |
|---|---|---|
| Isoattenuating fat-poor angiomyolipomas | Hounsfield unit –10 to 45 with no macroscopic fat variable contrast enhancement pattern | T2-weighted—homogeneously hypointense Chemical shift—signal loss Variable enhancement pattern |
| Angiomyolipomas with epithelial cysts | Hyperattenuating with variable cysts | T2-weighted—hypointense solid component Fat sat—variable signal loss Variable cysts |
| Epithelioid angiomyolipoma | Hyperattenuating with Hounsfield unit >45 May have cysts Heterogeneous contrast enhancement |
T2-weighted—hypointense May have cysts Heterogeneous contrast enhancement |
Renal Mass Management
Although the American Urological Association guidelines do not recommend biopsy of renal masses before resection, approximately 20% of renal masses smaller than 4 cm, most commonly oncocytomas or AMLs, are benign and may be unnecessarily treated. Noninvasive imaging plays a critical role in preventing overtreatment; however, accurate diagnosis of oncocytomas by CT or MRI is not possible. Classic AMLs are readily diagnosed using CT or MRI based on the presence of macroscopic fat. While fpAMLs are more difficult to diagnose in one meta-analysis, fpAMLs were diagnosed with a sensitivity of 83% and a specificity of 93% with MRI.12
Still, it is important to try to noninvasively diagnose these fpAMLs. Active surveillance has been advocated for renal masses <2 cm or when there are significant comorbidities or limited life expectancy. Imaging is also helpful in the management of masses that are highly suggestive of fpAMLs. As noted in one review, “radiologists influence management of solid renal mass and the decision to perform active surveillance by diagnosing masses that may be benign (eg, fat-poor AML).”13 In doing so, patients may avoid unnecessary surgery or other invasive interventions.
AML with TS
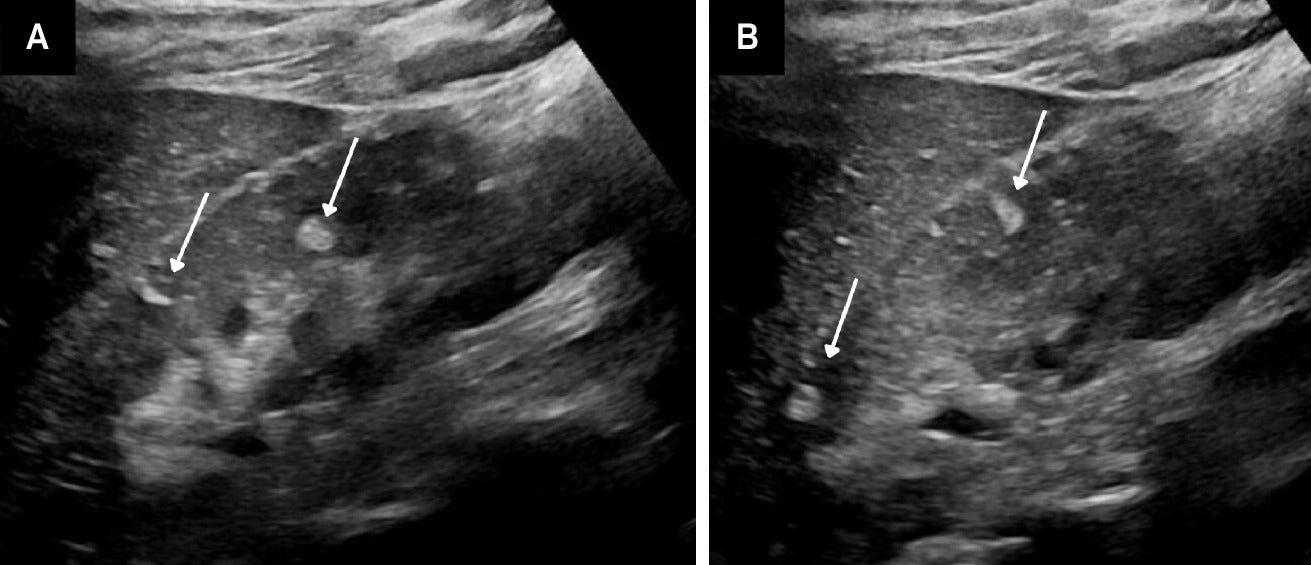
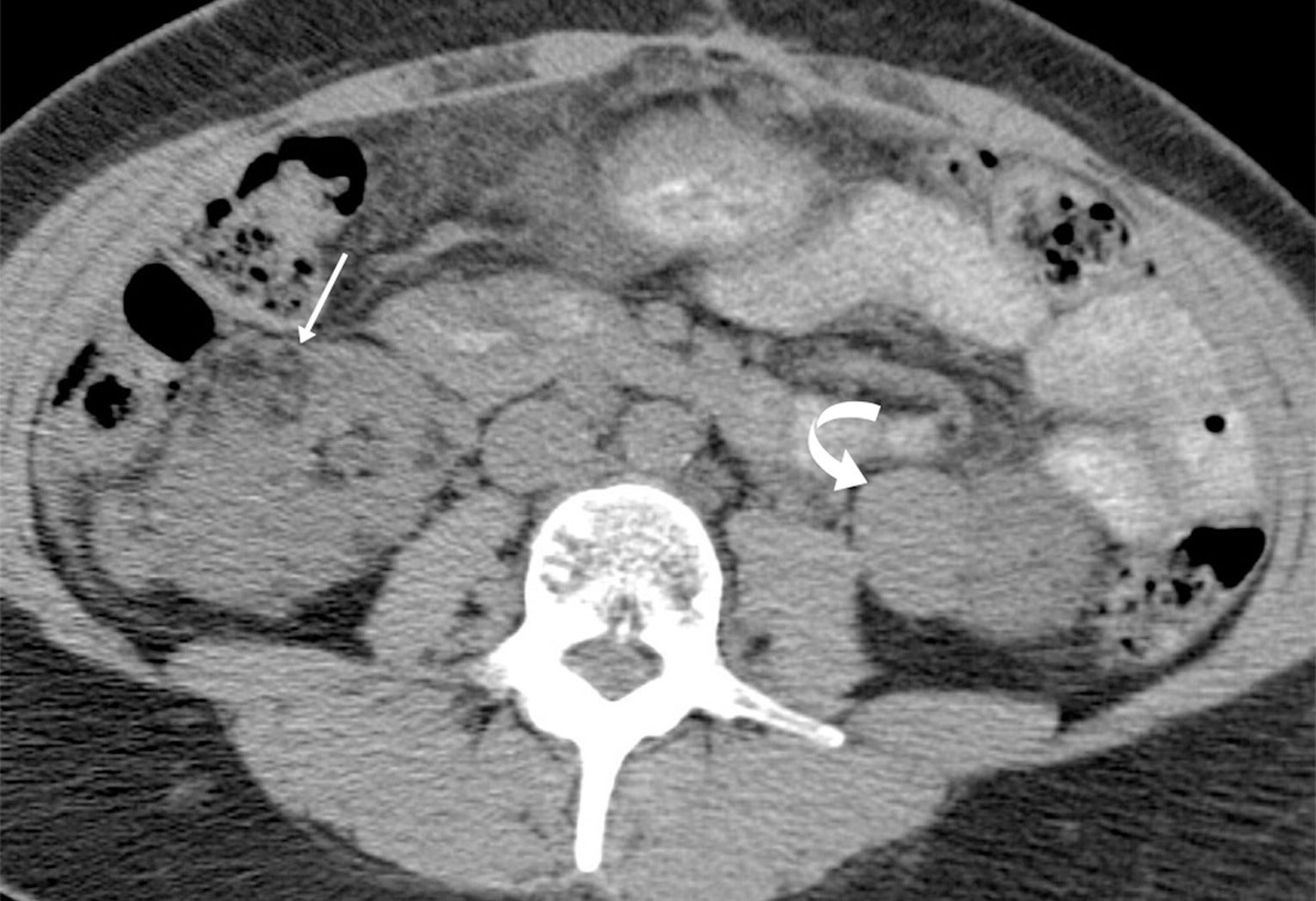
TS is a genetic disorder characterized by the presence of noncancerous tumors in multiple organs. Renal AML, usually the classic, fat-containing type, occurs in up to 75% of patients with TS. These tumors, which may occur at any age, are often multiple and bilateral ( Figure 3 ) and tend to enlarge with age ( Figure 4 ). Nonclassic subtypes, including fpAML, AML with epithelial cysts, and epithelioid AML, occur more frequently in TS than sporadic AML.4, 5 Close follow-up may be indicated. AMLs in TS tend to be larger and hemorrhage more frequently, necessitating treatment, often with catheter-based interventions for larger tumors (>4 cm) or those that have hemorrhaged.
Angiomyolipomas (AMLs) on US. Adolescent with tuberous sclerosis and multiple echogenic AMLs (arrows) within the right kidney (A). Similar masses were seen in the left kidney (B).
Angiomyolipomas (AMLs) on CT. Adult with tuberous sclerosis and multiple AMLs, most of which are classic with macroscopic fat (arrow) in the right kidney, but hyperattenuating fat-poor AML (curved arrow) in the left kidney.
AML with LAM
LAM is a rare, multisystem disorder, usually affecting females, that primarily presents with lung disease. The presence of pneumothoraces and chylous effusions can lead to respiratory failure. Though overwhelmingly pulmonary, LAM is a multisystem disorder that can result in meningiomas, chylous ascites, cystic masses, and renal AMLs. Renal AML occurs in fewer than 50% of patients with LAM, tends to be smaller than in TS, and hemorrhages less frequently.
AML with Epithelial Cysts and Epithelioid AML
The solid components of AMLs with epithelial cysts are hyperattenuating on CT, T2 hypointense on MRI, and associated with cysts, which may be multilocular. These are benign and typically managed with biopsy confirmation followed by observation.3
Epithelioid AML is rare and may be malignant. It is hyperattenuating on CT (HU >45), often shows heterogeneous contrast enhancement, and may have cystic components, hemorrhage, vascular invasion, necrosis, and/or metastases. Internal hemorrhage and necrosis are more common with epithelioid AML. On MRI, the solid components are T2 hypointense but more variable on fat saturation or chemical shift imaging.3 Owing to this, biopsy is often required to distinguish them from RCC.
Radiological Interventions
AMLs contain vascular tissues and may develop aneurysms, which can lead to hemorrhage, particularly as tumors enlarge ( Figure 5 ). Treatment approaches include percutaneous interventions ( Figure 6 ). Current treatment recommendations are predominately based on lesion size and patient symptoms.14 For the asymptomatic AML <4 cm diameter, serial imaging surveillance is typically sufficient.15 The presence of tumors larger than 4 cm, which carry higher risks of hemorrhage, and aneurysms >5 mm, meet the criteria for prophylactic intervention. Symptoms of hemorrhage may include acute abdominal pain ( Figure 5 ).16 AMLs may enlarge during pregnancy owing to hormonal effects, with an associated increased hemorrhage risk.17
Hemorrhage in large angiomyolipoma (AML). Adult with sudden onset of right flank pain. Coronal reformation contrast-enhanced CT showing left upper pole AML with angular interface (arrow). There is a large hematoma (H) that extends into the pelvis (B = bladder).
Embolization of large angiomyolipoma (AML). Coronal reformation contrast-enhanced CT showing AML with a large solid component (arrows) (A). Selective catheter angiography showing pooling of contrast (arrow) within the AML (B). Post-embolization catheter angiography showing near-complete occlusion of the vascular component of the AML (arrow) (C). Follow-up coronal reformation contrast-enhanced CT showing regression of the solid component of the AML, with some of the fatty component still visible (arrow) (D).
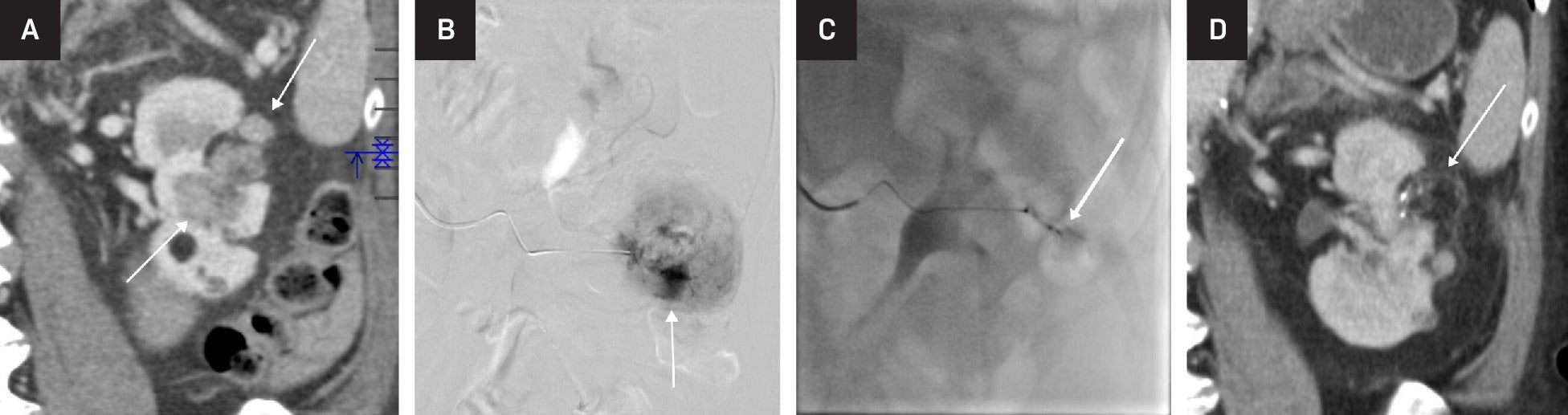
Arterial embolization and lesion ablation are common radiological interventions for AMLs. Embolization is preferred for larger tumors as it more effectively and directly addresses their vascular components. Arterial embolization may be used for prophylactic treatment of tumors >4 or >6 cm or those with aneurysms, and it is the treatment of choice when there is associated hemorrhage. Embolization is minimally invasive, with minimal blood loss and short hospital stay compared with surgical intervention.16
Radiofrequency ablation, including microwave and cryoablation, is a new treatment for AMLs <4 cm.14, 16 Ablation is performed under CT guidance using a percutaneous approach. Surgery remains an option in these cases but is more invasive and has been shown to have a higher rate of complications. Thus, surgery is usually reserved for larger lesions, but is associated with a higher rate of complications, including hemorrhage and urine leak,16 compared with embolization.
Summary
Most AMLs are sporadic, occur in females, are highly echogenic, and contain macroscopic fat detectable on CT and MRI. However, several subtypes exist, which the radiologist should be aware of, including the hyperattenuating and isoattenuating forms of fpAML, AMLs with epithelial cysts, and epithelioid AMLs. All of these have some imaging features that may allow them to be distinguished from RCC.
References
Citation
Chen AF, McGahan ;JP, FACR;1. New Trends in the Diagnosis and Management of Renal AML Subtypes: Part II. Appl Radiol. 2025;(4):.
doi:10.37549/AR-D-25-0083
October 1, 2025