New Trends in Diagnosis and Imaging Follow-Up of Renal Angiomyolipomas—Part I: Classic AMLs
CME credits available here.
Introduction
Classification of renal angiomyolipomas (AMLs) may initially seem simplistic. However, there have already been numerous attempts at pathological or imaging-based classification of these tumors, each of which tries to capture the nuances and variation among AML types.
The majority of AMLs are sporadic and asymptomatic and therefore most commonly identified as an incidental finding on imaging. However, AMLs often occur with tuberous sclerosis, a condition where benign tumors may be present in the brain, eye, heart, lungs, and kidneys. While exceedingly rare, AMLs may also be present with pulmonary lymphangioleiomyomatosis (LAM) ( Table 1 ).1
Etiologies of Renal Angiomyolipomas
| Occurrence | |
|---|---|
| Sporadic | Majority of angiomyolipomas, without associated symptoms |
| Tuberous sclerosis | Rare |
| Lymphangioleiomyomatosis | Very rare |
Abbreviations : ACR, American College of Radiology; CAR, Canadian Association of Radiologists.
The following approach attempts to provide a framework for understanding how the pathological features of AMLs translate to imaging features, which can be used for categorization and to differentiate AMLs from malignant renal cell carcinomas (RCCs), which may have similar appearances.
AML Tissue Types and Imaging
Angiomyolipomas are comprised of 3 tissue types: vascular, adipose, and muscular, each of which uniquely affects their imaging features and potential impact on clinical outcomes—for example, the vascular component of the AML may contribute to spontaneous hemorrhage. In rare instances, AMLs may contain benign epithelial cysts or malignant cells (as in the epithelioid type) ( Table 2 ).
Various Types of Renal Angiomyolipomas
| Type | Frequency |
|---|---|
| Classic | >90% with macroscopic fat |
| Fat-poor | |
| Hyperattenuating angiomyolipoma | 5% |
| Hypoattenuating angiomyolipoma | Rare |
| Fat-invisible | Rare |
| Angiomyolipoma with epithelial cysts | Very rare |
| Epithelioid angiomyolipoma (potentially malignant) | Very rare |
The presence of macroscopic fat is a key imaging feature of classic AMLs. While there are fat-poor or fat-invisible AMLs, where macroscopic fat is not present, that present a greater challenge for imaging diagnosis,1 as the muscular component of these masses contributes significantly to their imaging features (see Part II of this series), they are rare. In Part I of this 2-part series, we will detail some of the classic features of AMLs that contain macroscopic fat.
US Features
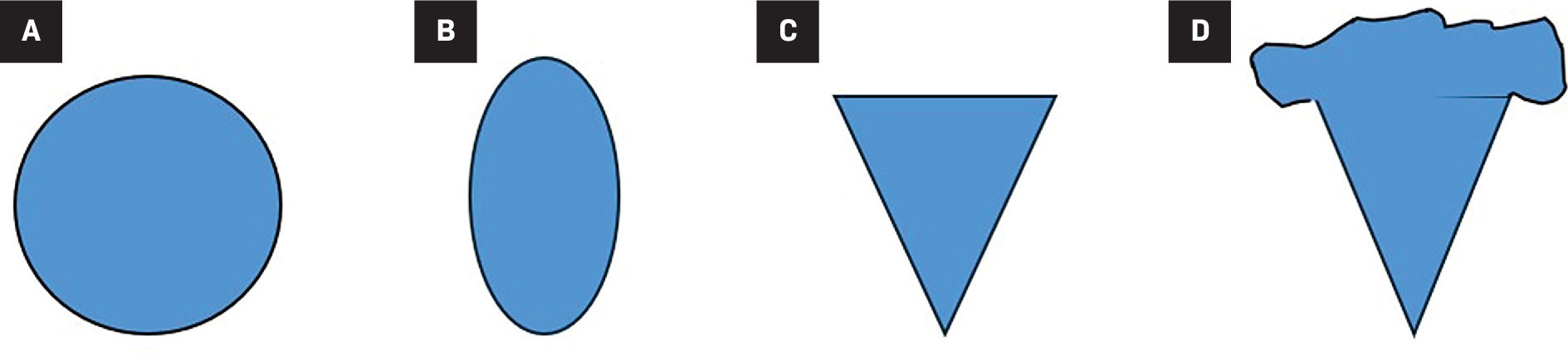
It is thought that the macroscopic fat in AMLs contributes to their echogenic imaging features on US. Research has shown that echogenic renal masses are usually AMLs or benign entities such as areas of infarction and/or scarring.2 However, an echogenic renal mass may also be an RCC,3 and this overlap creates a diagnostic dilemma. Recent studies have demonstrated characteristics that more reliably differentiate AMLs from RCCs on US, including the shape of the mass, whether it has an angular interface, presence of an “overflowing beer” sign, and an echogenic margin ( Figure 1 ).
Renal cell carcinomas are most commonly round in shape (A), while angiomyolipomas (AMLs) have variable shapes including oval (B), angular interface (C), and “overflowing beer” sign in some peripheral AMLs (D).
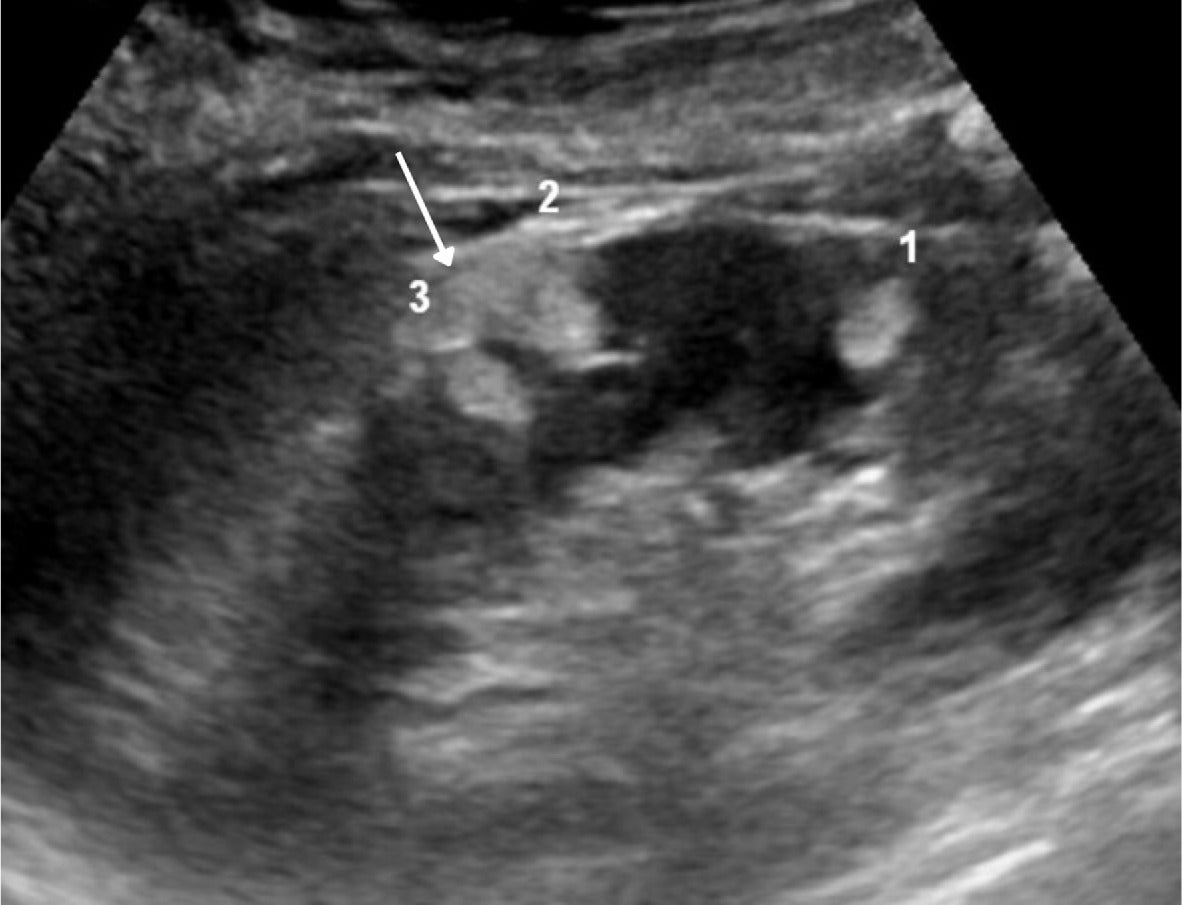
Sporadic AMLs may be multifocal, with different shapes ( Figure 2 ), compared with sporadic RCCs. For instance, in separate studies comparing endophytic and peripheral AMLs with RCCs, an oval shape was more common with AMLs than with RCCs, which are almost always round ( Figure 2 ).4, 5
Renal US in an adult with multiple angiomyolipomas, including 2 that are oval (1, 3) and one that is angular with “overflowing beer” sign (2) (arrow).
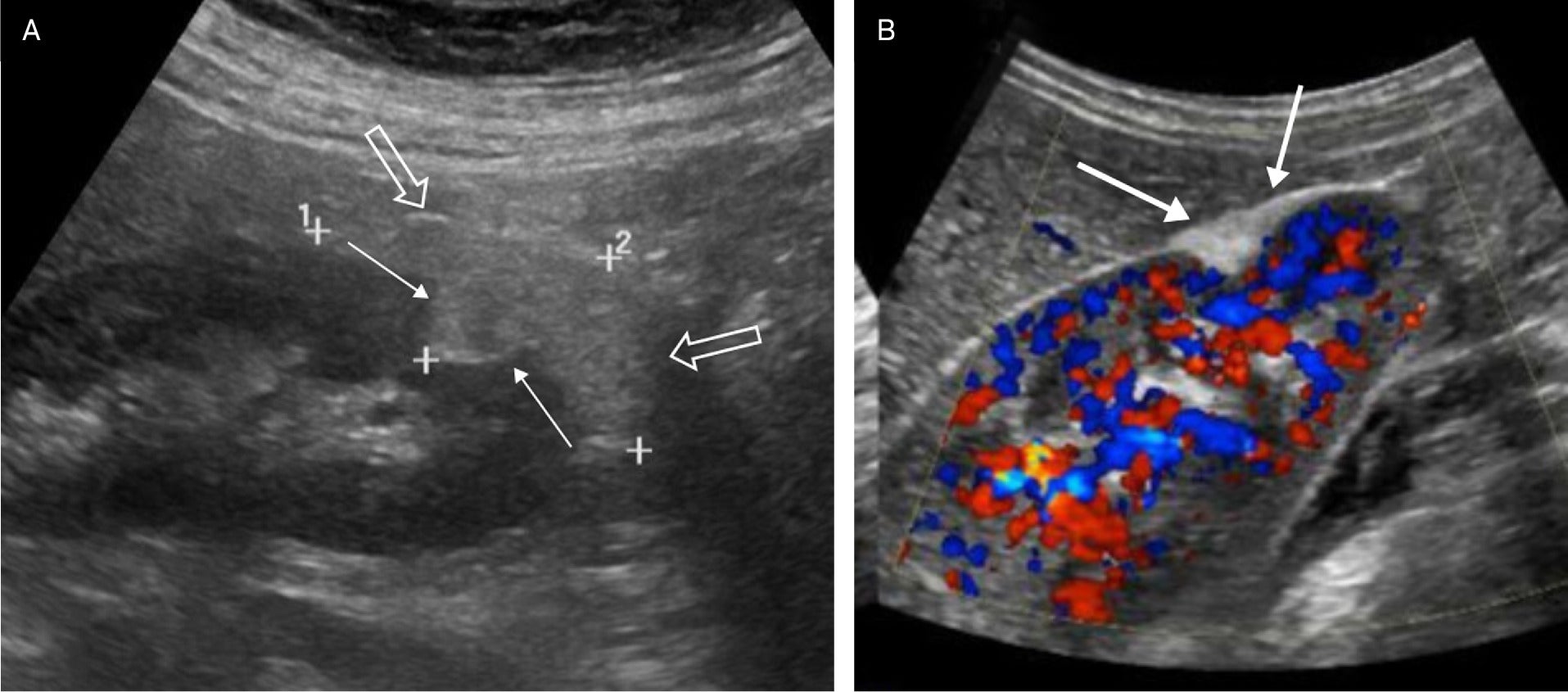
Endophytic AMLs were more likely to have an echogenic margin compared with RCCs. An angular interface was identified with peripheral AMLs and rarely with RCCs ( Figure 2 ). Similarly, an “overflowing beer” sign ( Figure 3 ) was only identified with peripheral AMLs and never with RCCs ( Table 3 ). Both studies showed that over 80% of the patients with sporadic AMLs were female.4, 5 AMLs occurring in the context of syndromes such as tuberous sclerosis are usually multiple and have similar shapes to sporadic AMLs.1
Renal US in an elderly patient with a large angiomyolipoma (AML) demonstrating an angular interface (solid arrows), but in addition the AML overflows the margins of the kidney as the “overflowing beer” sign (open arrows) (A). Renal color Doppler US image in an adult with peripheral AML demonstrating the “overflowing beer” sign (arrows) of the echogenic AML (B).
US Features to Help Distinguish Angiomyolipomas
| Renal Cell Carcinoma | Renal Angiomyolipoma | |
|---|---|---|
| Round | Very common | Present |
| Oval | Rare | Common |
| Angular interface | Rare | Present |
| “Overflowing beer” sign | Almost never | Present |
| Echogenic margin | Present | Common |
Researchers using US radiomics, with software that is not yet commercially available, have shown that some AMLs are more echogenic than RCCs.6 These authors compared the tumor to cortex echogenicity ratio and 18 other US texture features to differentiate echogenic AMLs from RCCs. They also found that RCCs were larger than AMLs, which may be another distinguishing feature.6
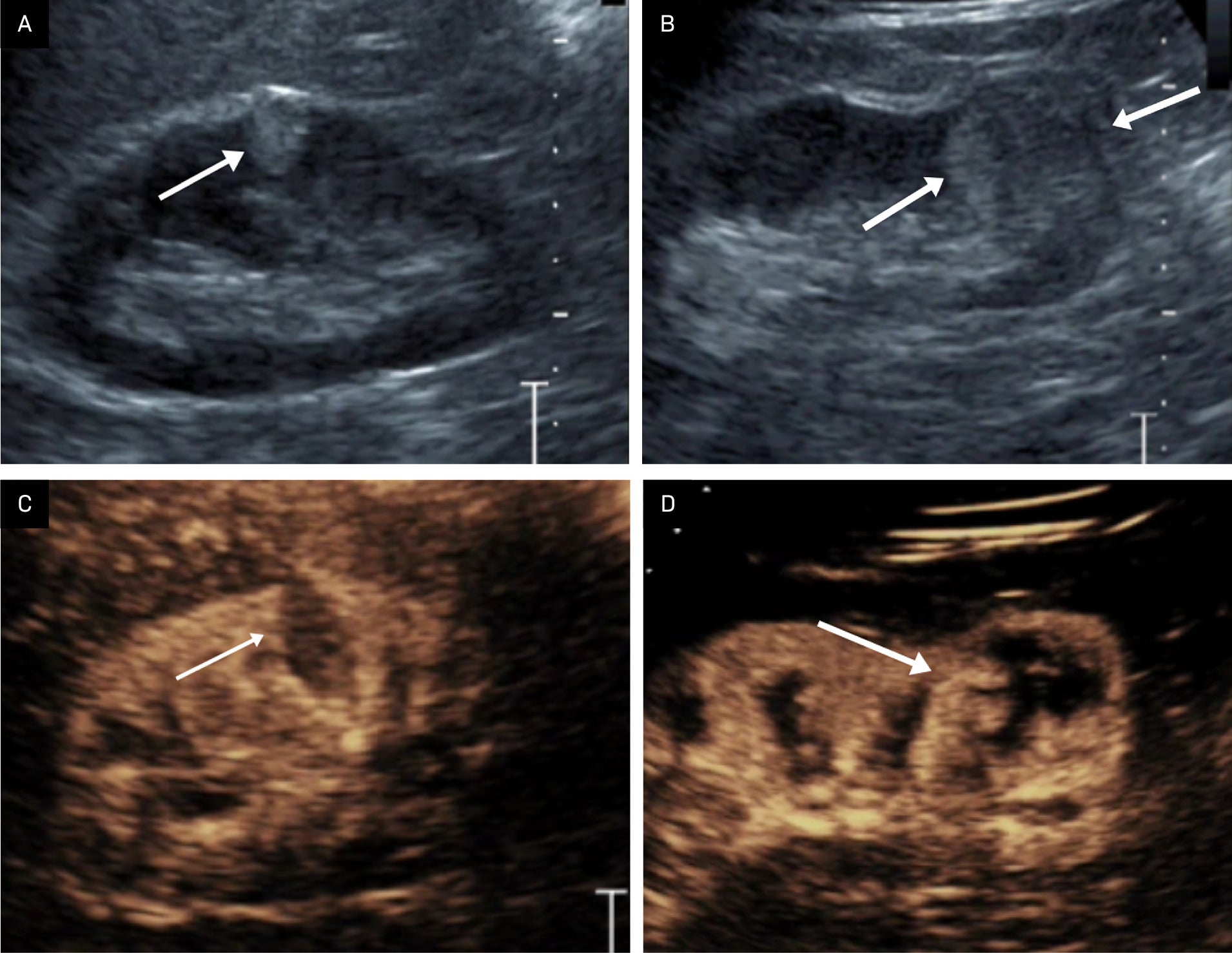
Power Doppler US, contrast-enhanced US (CEUS), and microvascular flow imaging are evolving technologies that have been shown to be helpful in diagnosing AMLs. For instance, Barr et al showed that CEUS can be useful in distinguishing echogenic renal masses from other masses ( Figure 4 ).7 Cao et al found CEUS features of fast washout and rim enhancement occurring with RCC had a sensitivity of 95% and a specificity of 91% in distinguishing RCCs from AMLs.8
Benefit of contrast-enhanced US. Renal US demonstrating an echogenic interpolar renal mass with an angular interface, corresponding to an angiomyolipoma (AML) (arrow) (A). Renal US showing a more rounded, lower pole renal mass corresponding to a renal cell carcinoma (RCC) (arrows) (B). Contrast-enhanced US showing renal parenchymal enhancement, but little enhancement within the AML (arrow) (C). Contrast-enhanced US showing that the mass enhances as great or greater than the renal parenchyma, with a slightly enhancing rim, indicating an RCC (arrow) (D). Figure is courtesy of Richard G. Barr, MD, PhD, of Northeast Ohio Medical University.
Imaging Follow-Up
There is agreement within much of the medical literature and among most organizations regarding how best to manage incidental echogenic renal masses greater than 1 cm in diameter. Thin-section CT should be considered as the next step in imaging evaluation when an echogenic mass is detected, as it can identify classic AMLs with macroscopic fat having Hounsfield units < 10.9 The consensus statements from the Canadian Association of Radiology (CAR) and the American College of Radiology (ACR) agree on the recommendations for echogenic masses greater than 1 cm,10, 11 but disagree on smaller echogenic masses. For example, the ACR’s statement indicates that masses smaller than 1 cm in diameter are so rarely malignant that they do not require follow-up,10 whereas the CAR statement recommends annual US for 5 years.11 Given the more recent studies analyzing the morphological features of these common sub-centimeter renal masses, then the recommendation of the ACR can be followed if features such as an echogenic margin, oval shape, angular interface, or “overflowing beer” sign are present.
CT/MRI: Classic AMLs
Most AMLs occur spontaneously in women and contain macroscopic fat. Renal AMLs are a common occurrence in patients with tuberous sclerosis and can appear in patients with LAM. The features of AMLs occurring with either tuberous sclerosis or LAM are similar to those of the various types of spontaneous AMLs.
Fat-rich AMLs are by far the most common type and are definitively diagnosed on CT and MRI. A classic CT feature of the fat-rich AML is CT attenuation of -10 Hounsfield units or less within the macroscopical fatty component ( Figure 5 ).11, 12 MRI features of fat-rich AMLs include increased signal intensity on T2-weighted MRI images, but this is not pathognomonic of AMLs. However, utilizing fat-suppression techniques allows the region of high signal intensity to be suppressed on these sequences, confirming the presence of macroscopic fat ( Figure 6 ). Likewise with chemical shift imaging, there will be an “India ink” artifact at the fat/water (renal parenchyma) interface in the same region as the high signal intensity on T2-weighted imaging ( Figure 6 ; Table 4 ).13, 14
Coronal contrast-enhanced CT of the abdomen shows a right lower pole mass with solid components and macroscopic fat with Hounsfield units < 10 (arrows), consistent with a large, fat-rich angiomyolipoma.
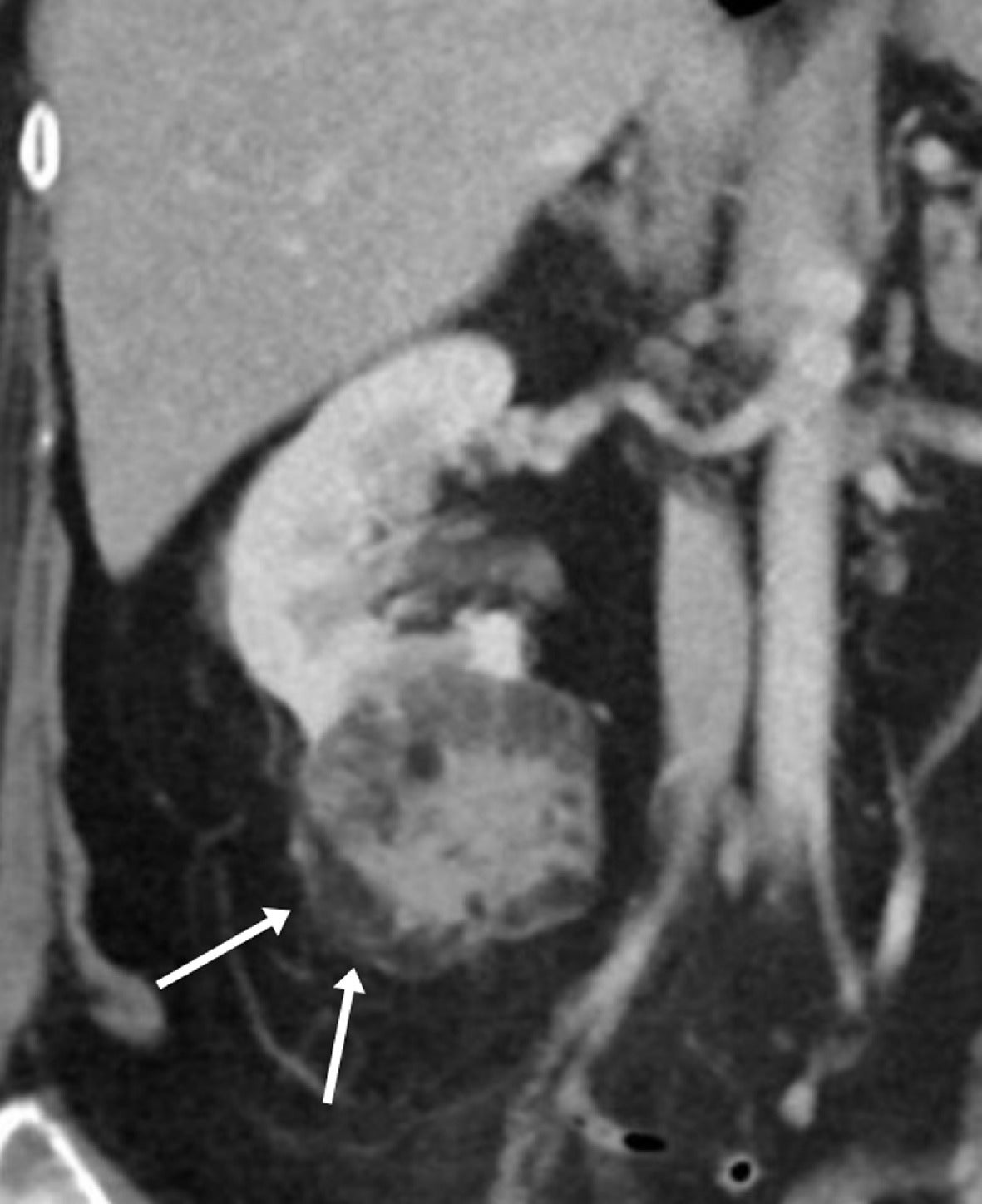
Classic angiomyolipoma (AML) on MRI. Coronal single-shot fast spin echo T2 image of a left renal peripheral AML (curved arrow), largely isointense to retroperitoneal fat (A). Axial T2 fat-suppressed image showing complete loss of signal intensity consistent with macroscopic fat in an AML (curved arrow) of the left kidney (B). Axial in-phase T1 MRI showing high signal intensity AML (curved arrow) in the left kidney (C). Axial out-of-phase MRI showing the “India ink” artifact as a dark line (arrows) at the interface of the macroscopic fat in the AML with the solid-appearing renal tissue (D).
CT and MRI Features of Classic (Macroscopic Fat) Angiomyolipomas
| CT | MRI |
|---|---|
| Fat attenuation < 10 Hounsfield units | T 2 W—hyperintense region due to macroscopic fat |
| No calcifications | Fat sat—signal drop out due to macroscopic fat |
| Occasional aneurysms | Chemical shift—India Ink artifact on contrast images due to macroscopic fat/water interface |
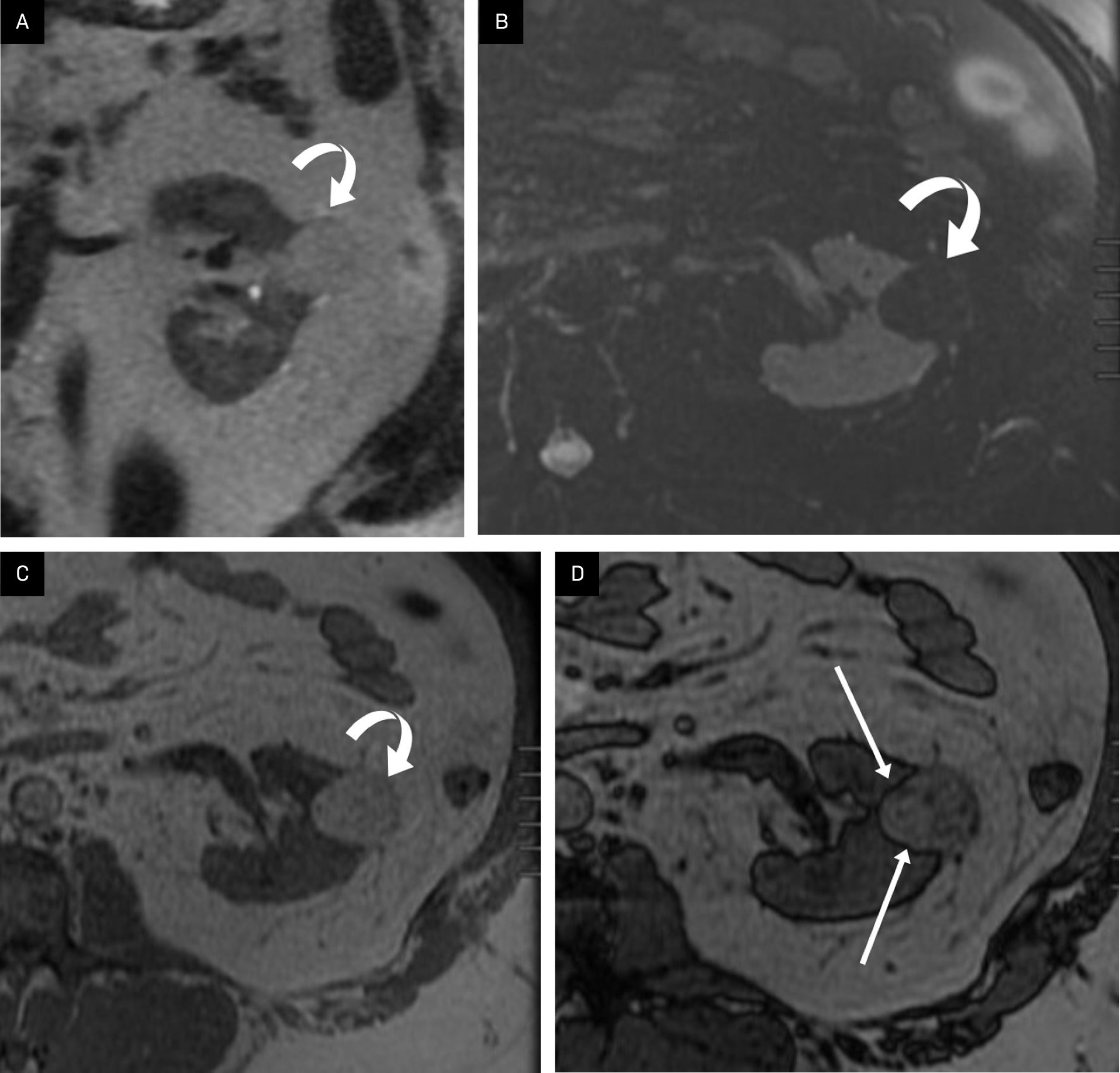
Morphological features of exophytic renal masses may be useful in distinguishing benign renal masses (cysts and AMLs) from RCCs on MRI.15 Verma et al showed that the presence of an angular interface of an exophytic mass > 2 cm on T2 MRI was a strong predictor of benignity.15 This angular interface may be identified with AMLs ( Figure 7 ) and with simple cysts ( Figure 8 ), making it a helpful feature in determining the benignity of a mass.
Angular interface on CT/MRI of angiomyolipoma (AML). An elderly patient with a peripheral AML on contrast-enhanced CT (A) and T1 LAVA post-contrast-enhanced MRI (B) showing angular interface (arrows).
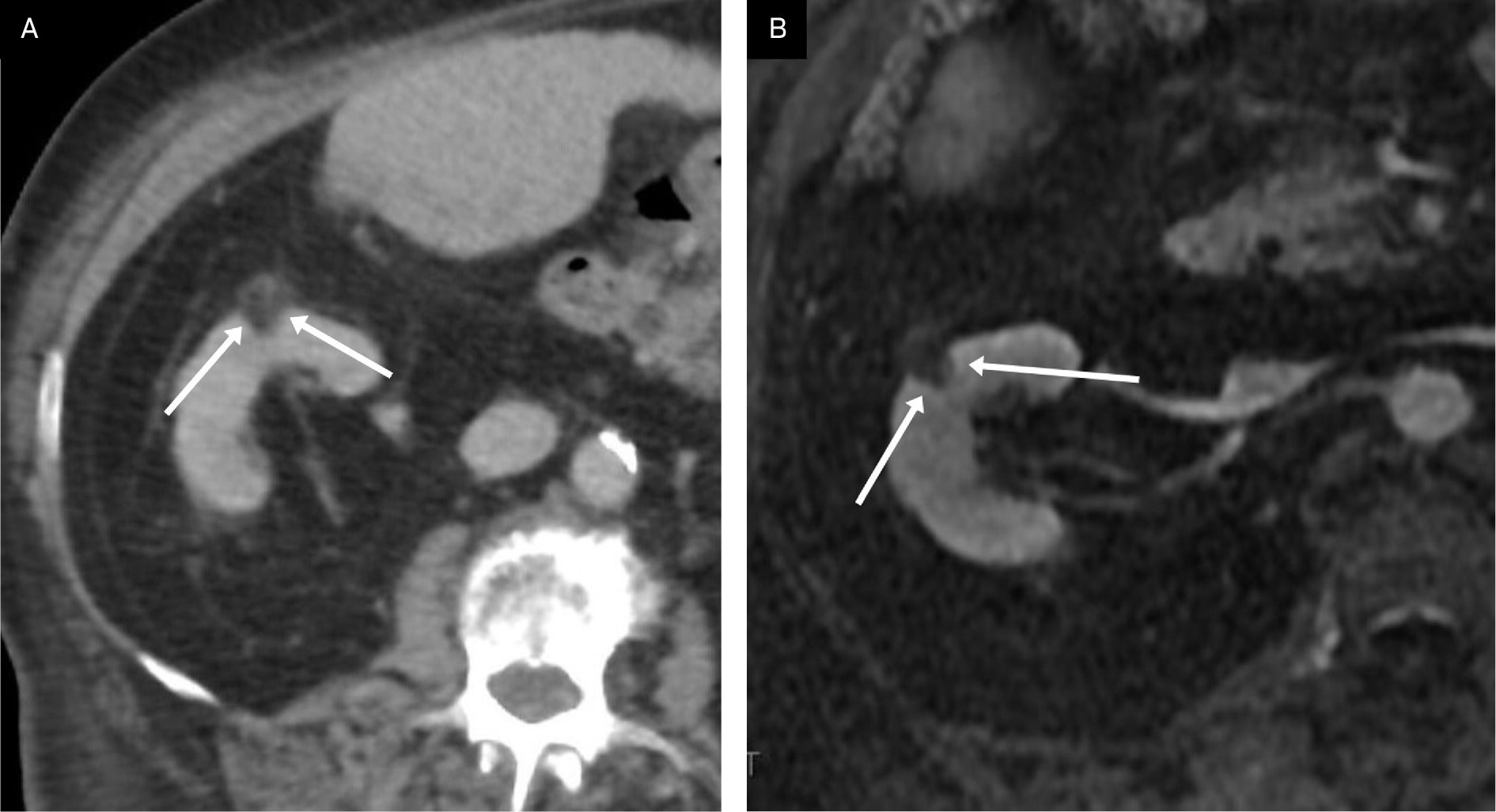
An elderly patient with a simple right renal cyst demonstrating an angular interface (curved arrow). Also note smaller peripheral hypodensity with an angular interface (arrow) probably representing an additional small cyst.
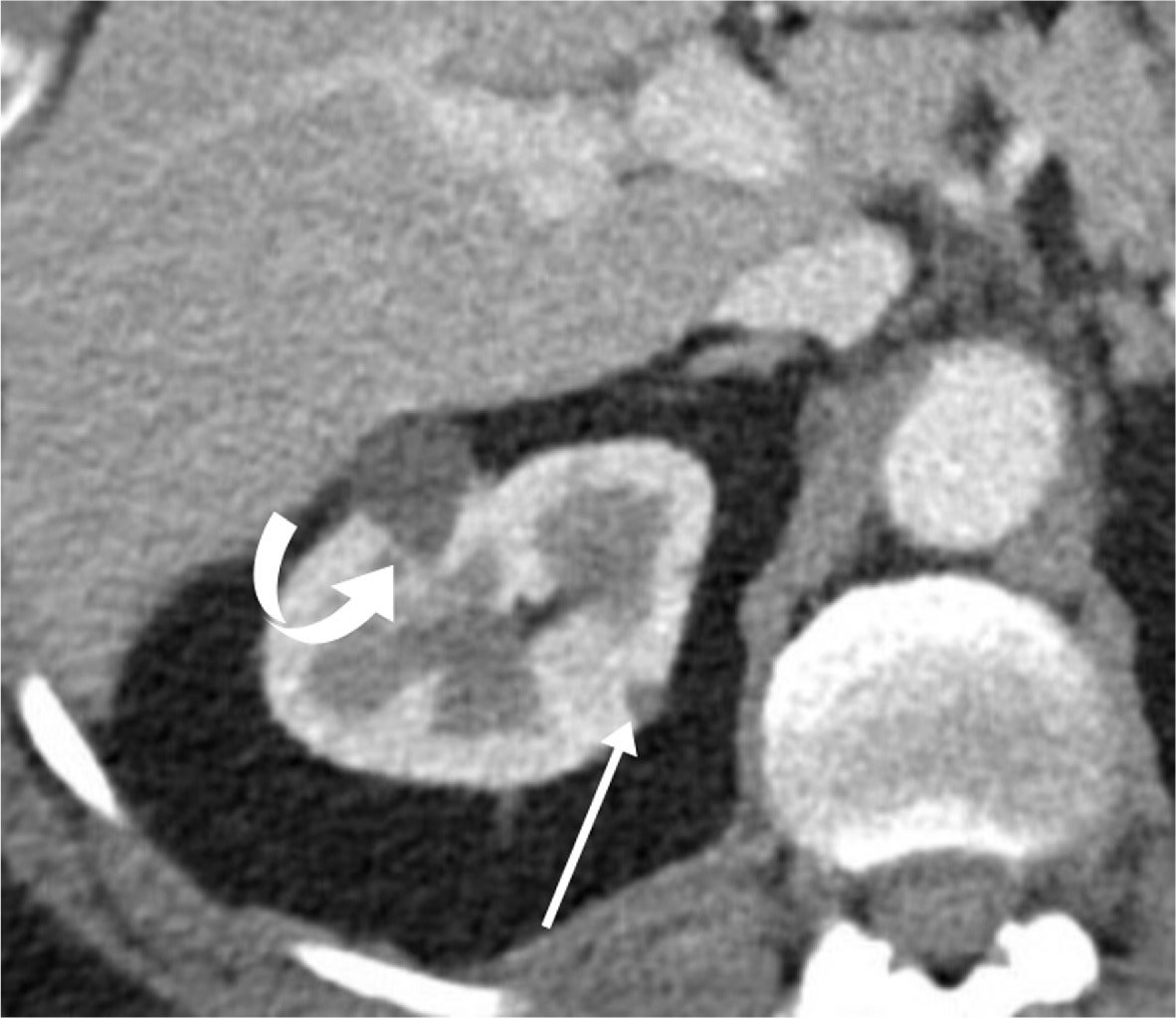
Other distinguishing features include intratumoral calcifications and aneurysms. Intratumoral calcifications are rare within AMLs but may be present with RCCs. Conversely, intratumoral aneurysms may occur with classic AMLs but are rare with RCCs.
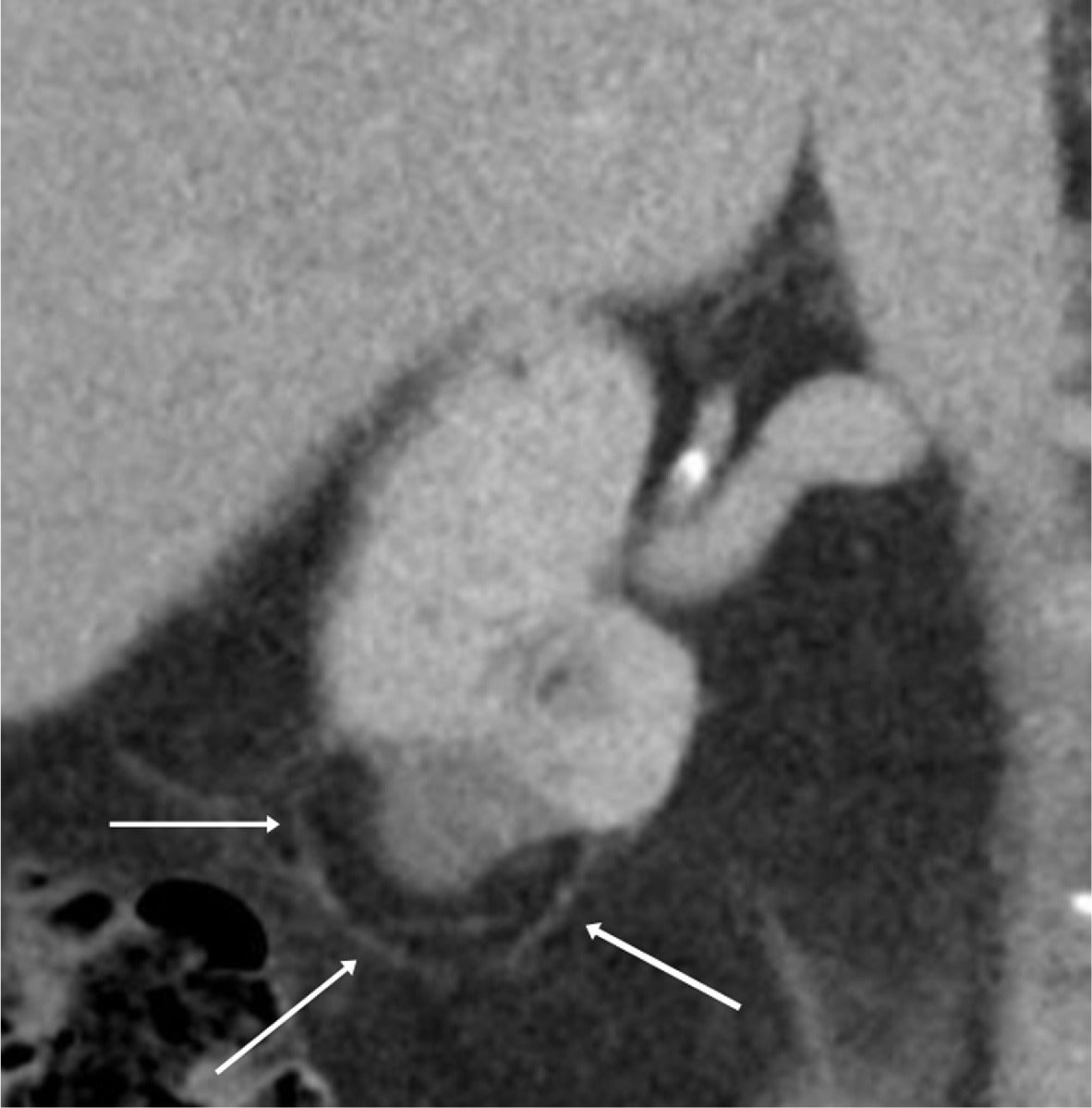
Less common renal and/or retroperitoneal masses may also have a macroscopic fatty component. For instance, retroperitoneal liposarcomas, when discovered, are typically large, with an average diameter of 21 cm in one series.16 They less commonly have an associated renal parenchymal defect than large AMLs, and liposarcomas are more often poorly marginated, sometimes displacing the kidney ( Figure 9 ).16 Patients treated for RCC by radiofrequency ablation may have encapsulated retroperitoneal fat “pseudomass” at the site of ablation ( Figure 10 ), not to be mistaken for a fat-containing neoplasm.17
An elderly patient with a large retroperitoneal liposarcoma (L) that is poorly encapsulated and displaces the left kidney (arrow) without a capsular defect.
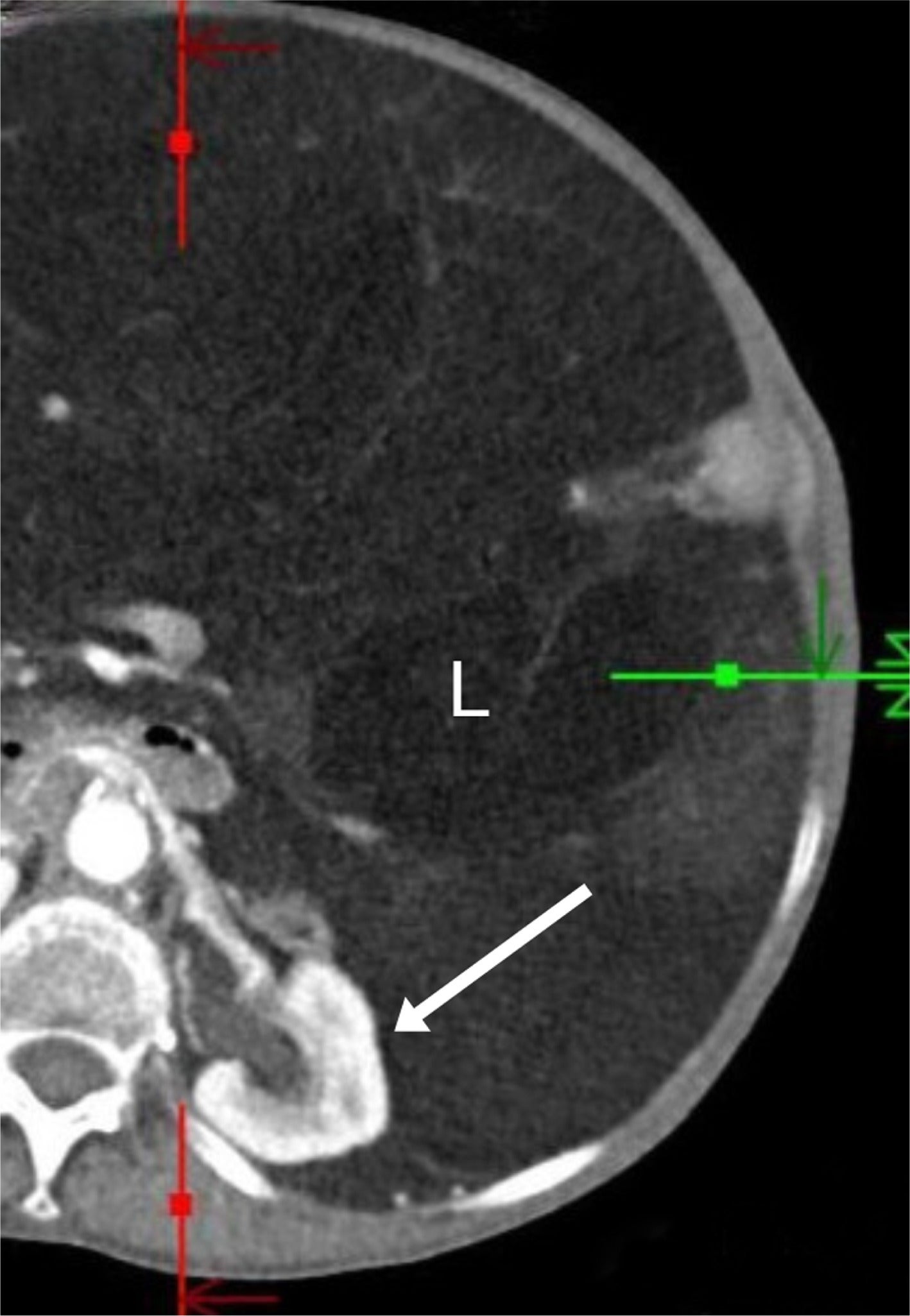
An elderly patient with a 1-year follow-up contrast-enhanced CT after radiofrequency ablation of a clear cell renal cell carcinoma, showing an avascular mass with surrounding encapsulated fat (arrows).
Conclusion
Understanding the histology and imaging features of classic AMLs can aid in the distinction of these lesions from other primary renal neoplasms, most commonly RCC. While emerging radiomics and advanced US techniques can provide increasingly valuable information, CT and MRI remain the mainstay for imaging characterization of these masses.
References
Citation
McGahan JP, Chen AF.New Trends in Diagnosis and Imaging Follow-Up of Renal Angiomyolipomas—Part I: Classic AMLs. Appl Radiol. 2025; (2):6-11.
doi:10.37549/AR-D-25-0076
April 30, 2025