Practical issues in abdominal PET/CT
Images

Dr. Blake is the Director of Abdominal PET/CT, Dr. Slattery is a Clinical Fellow, Dr. Sahani is the Medical Director of CT services and, at the time this article was written, Dr. Kalra was a Research Assistant, Department of Radiology, Division of Abdominal Imaging and Intervention, Massachusetts General Hospital, Boston, MA. Dr. Kalra is now the Director of CT Research, Emory University, Atlanta, GA.
Imaging continues to play a major role in the management of oncologic patients. Most imaging modalities yield purely anatomic and morphologic tumor detail without addressing tumor metabolism. The advent of positron emission tomography (PET) with 18Ffluorodeoxyglucose (FDG) has provided tumor-related qualitative and quantitative metabolic information critical to patient diagnosis and management. PET enables the detection of increased metabolic activity in tissue that can appear morphologically normal on other imaging modalities. It can also assist in the differentiation of benign from malignant lesions and in the imaging follow-up of cancer patients following surgery, radiation, or chemotherapy. 1-3 PET, however, is limited by relatively poor spatial resolution, whereby accurate anatomic localization of foci of increased metabolic activity can be difficult or impossible. This is particularly the case in the abdomen, where there may be a lack of identifiable anatomic structures.
One of the most exciting technological advances in recent years is the clinical application of combined PET and computed tomography (CT) scanning. First described by Beyer et al, 4 PET/CT provides high-resolution cross-sectional information from CT coregistered with metabolic information from PET. The imaging data are acquired using a combined scanner during a single examination and are subsequently fused. The CT images are used for more precise and rapid attenuation correction of the PET data. The combination of CT detail with PET data leads to more assured anatomic localization of areas of increased metabolic activity (Figure 1).
In addition, both modalities have further individual strengths that are highly complementary. PET can be used with different radiotracers to provide other useful biologic information, while CT provides valuable multiplanar information regarding the morphological features and attenuation values of lesions as well as oral and intravenous (IV) contrast behavior. In addition to reducing the PET imaging time per patient from 45 to 60 minutes with a conventional dedicated PET scanner to 15 to 30 minutes, a PET/CT scanner also reduces the number of equivocal PET and CT inter-pretations. 5,6 PET/CT offers many other possible advantages for improved patient care. These include improved diagnosis and staging of abdominal cancers, more accurate identification and localization of disseminated malignancy and recurrent disease, and planning and monitoring of surgical and adjuvant therapy. 4,7
The artifacts and pitfalls related to interpretation of independent PET and CT images are well documented, and more are being reported as clinical experience grows. 8,9 The combined modality PET/CT provides its own specific pitfalls and artifacts. 10 It is essential that a working knowledge of these pitfalls as well as the principles of PET/CT be acquired in order to ensure accurate image interpretation and to optimize patient care. This review will highlight some of the problems regarding abdominal PET/CT performance and address some specific protocol and interpretation issues.
Abdominal PET/CT protocol
The introduction of CT-based attenuation correction and its integration with PET necessitates different PET/CT scanning protocols. The 2 general approaches adopted for PET/CT scanning are essentially to limit the CT to simply perform faster attenuation correction, or to make full use of the CT for both attenuation correction and diagnosis and coregistration. 11 The former approach employs the lowest possible CT radiation dose to achieve attenuation correction. The latter approach allows diagnostic quality CT to be achieved with a standard radiation dose. The specific indications and protocols for low or standard radiation dose CT have yet to crystallize. We combine the 2 approaches by employing a low-dose CT scan without IV contrast to provide attenuation information and a fully diagnostic contrast-enhanced scan acquired at standard radiation dose immediately following the PET acquisition. Other contentious issues include the reimbursement for the study, the validation of indications for use, and who should interpret the exam. 12,13
Although recent studies have shown that oral and IV contrast media can be administered for the diagnostic CT to aid in lesion localization and support characterization, modifications are necessary to avoid image artifacts in the PET images and to ensure appropriate attenuation correction (AC). 14-16 Beam-hardening artifacts can occur from metallic orthopedic and dental implants, which affect CT-based attenuation correction of PET images. 17-19 In addition, mismatch of internal organs due to breathing movements and inconsistent patient positioning must be minimized to allow precise PET/CT coregistration in abdominal studies. 20,21 Normal "free" breathing or normal expiratory phase for acquisition of CT images has been found to be more suitable than maximum inspiratory or maximum expiratory phases for coregistration. Breath-hold imaging, however, clearly confers significant advantages in CT image quality. Our dual CT acquisition protocol described above allows us to acquire, at a minimum, the contrast-enhanced scan during a breath-hold.
Attenuation correction
In PET/CT scanners, the PET emission data can be corrected for photon attenuation using the CT image to generate a 511-keV attenuation map. This is achieved by algorithmically converting the Hounsfield units (HU) in CT to attenuation coefficients appropriate for energies of 511 keV. All manufacturers of PET/CT scanners incorporate CT-based AC algorithms in their systems; in fact, for some PET/CT scanners, it is the only option offered. This technique has several advantages. First, there is less statistical noise from the CT data compared with transmission data acquired by a radionuclide source, such as 68Ge in standard PET scanning. Second, scan time for the acquisition of CT data is much shorter than radionuclide sources, thus reducing overall scan time by 15 to 20 minutes. Finally, the need for PET transmission hardware is eliminated.
However, there is a potential risk of overestimating the true tracer activity with CT-based AC. Nakamoto et al 22 has shown that the measured activity with CT-based AC was overestimated by an average of 11% in bone and 2.1% in soft tissue when compared with 68Ge-based AC. Thus, it is important to take this into account when interpreting quantitative or semi-quantitative (standardized uptake value [SUV]) changes between a study performed with CT AC and a study performed with 68Ge AC. Attenuation correction using CT data may lead to some artifacts that are caused by overcorrection of photopenic areas corresponding to high-attenuation structures on CT. This incorrectly renders such areas hypermetabolic on the AC-PET images. Some studies have shown that the use of high-density positive oral and IV contrast media may lead to AC artifacts in the PET image. 23-26
Antoch et al 27 reported that artifacts from IV contrast are limited to the transient bolus passage of undiluted contrast through the great vessels in the thorax. Artifacts from oral positive contrast agents can also arise where there are focal concentrations of high-density contrast, such as bar-ium. 24 Low-density oral contrast usually does not result in major artifacts. In clinical practice, Dizendorf et al 25 reported only a 4% overestimation of SUVs in relation to positive oral contrast media, suggesting a negligible effect. Where focal accumulations of positive oral contrast media do give rise to artifacts, these can be resolved by viewing the non-AC corrected images. In an effort to avoid contrast-induced artifacts, negative oral contrast media are being evaluated. 28 Metallic prosthetic implants (such as hip replacements, intrauterine contraceptive devices, or surgical clips) can lead to beam hardening on CT, with consequent AC artifact on the PET image. It is important to view the uncorrected PET images to avoid false-positive interpretation, particularly when there is a question regarding periprosthetic inflammation or infection 18 (Figure 2).
Algorithms are being developed to allow for this overestimation of activity by CT AC and may minimize these effects in the future. Availability of diagnostic CT is extremely valuable in situations in which tumor is not 18F-FDG-avid, since contrast agents facilitate tumor detection and characterization. Diagnostic CT can also reveal clinically pertinent non-neoplastic conditions, such as aortic aneurysms and bowel obstruction. Therefore, the use of oral and IV contrast media can augment CT performance beyond mere anatomic correlation and attenuation correction for PET. 14,15
Interpretation issues
In general, false-positive FDG uptake can occur in PET/CT in relation to granulomatous disease or inflammation. Some benign tumors, such as colonic adenomas and fibroids, may also demonstrate intense FDG uptake. False negatives can arise in tumors that are either small or non-FDG-avid. These include some neuroendocrine tumors, renal cell carcinoma, and certain types of lymphoma. Many previous studies have demonstrated that PET is more specific than CT for staging of posttreatment lymphoma patients. 29 However, there are subtypes of lymphoma that are poorly avid on PET, including, most notably, marginal zone lymphoma (of which mucosa-associated lymphoid tissue [MALT] lymphoma is a subtype) and peripheral T-cell lymphoma. Therefore, CT may play a more important role in the staging of these patients at diagnosis and follow-up. 30 Some mucinous and low-grade tumors are also known to be poorly FDG-avid. 5 High neighboring background activity can also lead to obscuration of FDG uptake.
It is hoped that misinterpretation may be avoided by the ability to accurately attribute FDG activity to the correct abdominal structure. Radiotracer uptake within the gastrointestinal tract is highly variable (Figure 3). The esophagus generally does not demonstrate increased uptake in the absence of inflammation or malignancy. Homogeneous uptake in the stomach wall is relatively common, however. Small-bowel uptake is variable but usually of low grade. Uptake within the colon may be quite avid, particularly in the cecum/ascending colon and within the rectosigmoid region. This usually has a recognizable linear appearance. Focal large-bowel activity greater than hepatic activity is unusual and should alert the radiologist to the possible presence of pathology. A thorough regional review of the coregistered CT images is warranted to look for focal masses or adjunct signs of inflammation; one must bear in mind that peristalsis, patient motion, and breathing may lead to misregistration artifact. Preferably, this should be a diagnostic CT with oral and IV contrast media. In the absence of corresponding CT findings, focal intense colonic activity on PET still warrants further investigation. The exact cause of the variable FDG uptake in the intestinal tract is as yet unclear. Animal studies have shown that the majority is due to intestinal mucosa or bowel contents. 31
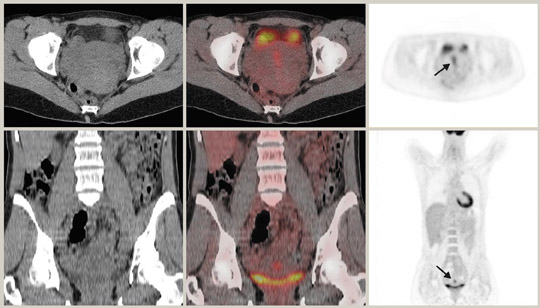
Although 18F-FDG is an analogue of glucose, unlike glucose, it is not reabsorbed by the renal tubules. Thus, any part of the urinary tract can show increased activity. In particular, dilated or redundant ureters as well as bladder diverticula can be confounding findings. 32 It is beneficial to minimize urinary stasis within the renal collecting system, ureters, and bladder, particularly when there is pelvic pathology. Good hydration and regular voiding can achieve this. Some authors advocate the use of IV diuretics 33,34 or bladder catheterization. 35 Care must be taken when interpreting PET images in the context of renal cell carcinoma, as a recent study has reported only 60% sensitivity. 36
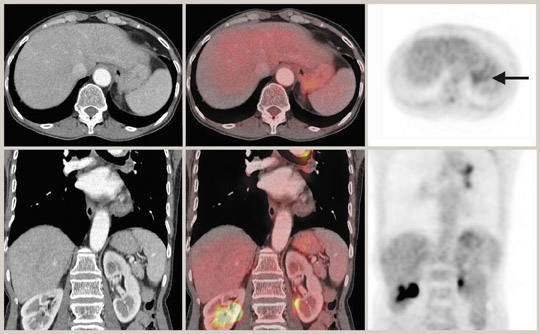
Liver activity is usually mildly intense with a uniformly mottled appearance. Caution must be exercised when interpreting liver dome lesions, as these can be erroneously projected in the lung base due to respiratory artifact. 37 In addition, hepatomas are known to be poorly FDG-avid. 38,39 Splenic activity is usually less than liver on FDG-PET, and CT can be of particular help with splenules that can cause some confusion on PET imaging alone. Some studies have shown that very early stage pancreatic cancers can give rise to false-negative results with FDG-PET, thus suggesting a major limitation of FDG-PET imaging alone in the detection of pancreatic cancer. 40 False-positive FDG-PET results for pancreatic cancer can occur in chronic active pancreatitis and autoimmune pancreatitis. 41 Similarly, focal uptake of the tracer compound by inflamed parenchyma and irradiated tissues may be indistinguishable from pancreatic malignancy. 42 There have also been reports of ocal FDG uptake caused by portal vein thrombosis, hemorrhagic pseudocysts, peripancreatic lymph nodes, and retroperitoneal fibrosis. 42,43 CT can be helpful in these situations by identifying the cause of the increased FDG uptake.
Adrenal adenomas are relatively common in the general population (2% to 9%) and occur in up to 5% of abdominal CT scans. 44 The majority of adrenal lesions can be characterized by CT criteria. Approximately 70% of adrenal adenomas contain sufficient intracytoplasmic fat to lower the CT attenuation to ≤10 HU. 45-48 Adrenal lesions can be further characterized by washout characteristics on delayed CT. 49-51 Characterization of adrenal lesions by FDG-PET depends on increased glucose metabolism in malignancy. Several series have investigated the ability of FDG-PET to distinguish between benign and malignant adrenal lesions. In a series of 50 adrenal lesions, Mijin et al 52 used background liver activity as a baseline. Lesions with activity lower or much greater than liver activity could be confidently diagnosed as benign or malignant, respectively. Lesions with slightly increased activity relative to liver were classed as indeterminate. Other authors have reported up to 100% sensitivity and specificity for FDG-PET in distinguishing benign from malignant lesions. 53,54 False-positive scans do occur in FDG-PET adrenal imaging, particularly in relation to pheochromocytoma, adrenal hyperplasia, angiomyelolipoma and in approximately 5% of adenomas. 55,56 PET/CT combines both the attenuation characteristics and the metabolic activity of adrenal lesions in one examination and should provide information that is diagnostic for most of the cases encountered.
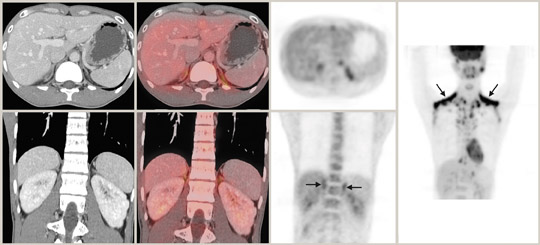
The integrated PET/CT information may be particularly useful in the rare event of collision tumors when an adenoma and metastasis coexist. Brown fat with a perinephric distribution is another potential pitfall of PET/CT in adrenal imaging because of increased FDG up-take (Figure 4). The characteristic brown-fat pattern is more commonly recognized in the neck and supraclavicular regions but can also be seen in the paraspinal and periadrenal regions. Brown fat is considered a vestigial organ of thermogenesis that utilizes increased glucose and is sympathetically innervated and driven. The pattern is more common in thin patients and during the winter months. Awareness of this entity together with careful CT correlation usually results in correct diagnosis. 57
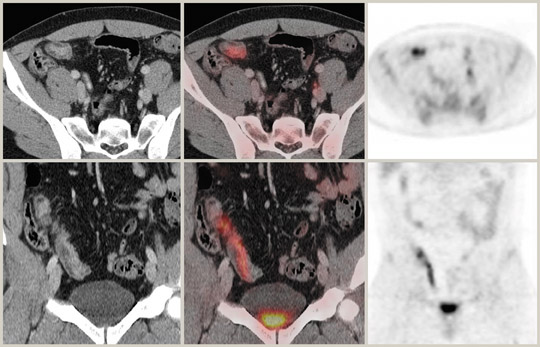
PET/CT is extremely useful in characterizing lesions in the pelvis. There are some pitfalls to be wary of, however. Endometrial uptake of tracer is seen in biphasic peaks at ovulation and menstruation in premenopausal subjects (Figure 5).
Postmenopausal endometrial uptake is abnormal. Ovarian uptake may be physiological in premenopausal subjects but is associated with malignancy in postmenopausal women. 58 PET and CT can be complementary in displaying bony abnormalities, with PET excelling in marrow abnormalities and CT performing better with cortically based lesions. The diffuse increase in bone marrow activity seen following colony stimulating factors is now well recognized. Paget's disease and fibrous dysplasia may show increased uptake but these entities can usually be recognized on CT. A dedicated PET/CT workstation is mandatory for optimal viewing of the abdominal and pelvic images. Reviewing the CT data using appropriate window settings and examining the displays of both the attenuation-corrected and noncorrected PET data serve to provide a comprehensive, integrated interpretation.
Conclusion
Although the PET/CT scanner clearly represents a major technologic advance, the alliance of functional imaging with structural imaging raises a number of controversial issues. Protocol and interpretation issues include examination reimbursement, validation of indications for use, who should interpret the examination, specific indications and protocols for low radiation dose CT, and suitability and timing of oral and IV contrast. Recent PET/CT publications have been highly encouraging, but larger prospective studies will be necessary to establish the optimal hybrid scanning protocols. We have outlined some specific performance and interpretation principles, including some that are still in development. Applying sound principles and staying abreast of advances in this exciting new modality are necessary requirements to harness the full diagnostic power of abdominal PET/CT.