Percutaneous and Transvenous Liver Biopsy
Images
CME credits are available for this article here.
Interventional radiology plays a key role in diagnosing, managing, and determining the prognosis of patients with liver pathology. Together with clinical and laboratory findings (aspartate or alanine aminotransferase, alkaline phosphatase), pathological evalua- tion following image-guided liver biopsy allows for histological characterization of disease.1
Indications for Liver Biopsy
Image-guided biopsy is useful in a number of ways with respect to diagnosing, treating, and managing patients with liver conditions. These include:
- Differentiating drug-related hepatotoxicity from autoimmune hepatitis in the absence of previous hepatotoxicity warnings.2
- Assisting in the diagnosis of hereditary conditions such as Wilson disease, A1-antitrypsin-1 deficiency, and hereditary hemochromatosis. In Wilson disease, a hepatic copper content > 250 μg/g has been reported as the best biochemical evidence for diagnosis;3 hereditary hemochromatosis can be diagnosed based on iron distribution within the tissue.4
- Identifying drug-induced fibrosis secondary to treatment with methotrexate; pre- and post-treatment biopsies, as well as follow-up biopsies, are recommended for patients after each accumulated dose of 1.5 grams.2
- Grading and staging of non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, chronic hepatitis, and many other associ- ated illnesses.2 In chronic hepatitis C (or persistent hepatitis B) patients, biopsy results help to determine the need for antiviral therapy. It is also recommended that biopsies be acquired every two to three years for assessment of disease progression.2 Based on recommendations by the American Association for the Study of Liver Disease (AASLD), the diagnostic and prognostic ability of liver biopsy for advanced fibrosis in patients with chronic hepatitis C infection cannot be achieved by noninvasive testing alone.2
- Staging for prognostic values and offering insight into physiological response to drug treatment and management regimens.2
- Assessing the acuity and degree of rejection In the setting of liver transplantation.2,5
- Identifying unexplained abnormalities of liver testing or hepatomegaly, hepatic neoplasms and lesions, infections, unexplained cholestasis, metabolic and genetic disorders, and fever of unknown origin.2
Percutaneous Liver Biopsy
Percutaneous liver biopsy (PLB) has been used to identify hepatic pathology since the first core biopsy was performed in 1880 by Paul Ehrlich in Germany and was adapted as a staple in histological liver diagnostics in 1957 by Menghini.6,7 The technical aspects of PLB differ from that of transvenous liver biopsy (TVLB). In the setting of non-image-guided PLB, the area of maximum dullness can be identified by percussion over the right hemithorax between the sixth and ninth intercostal space.8
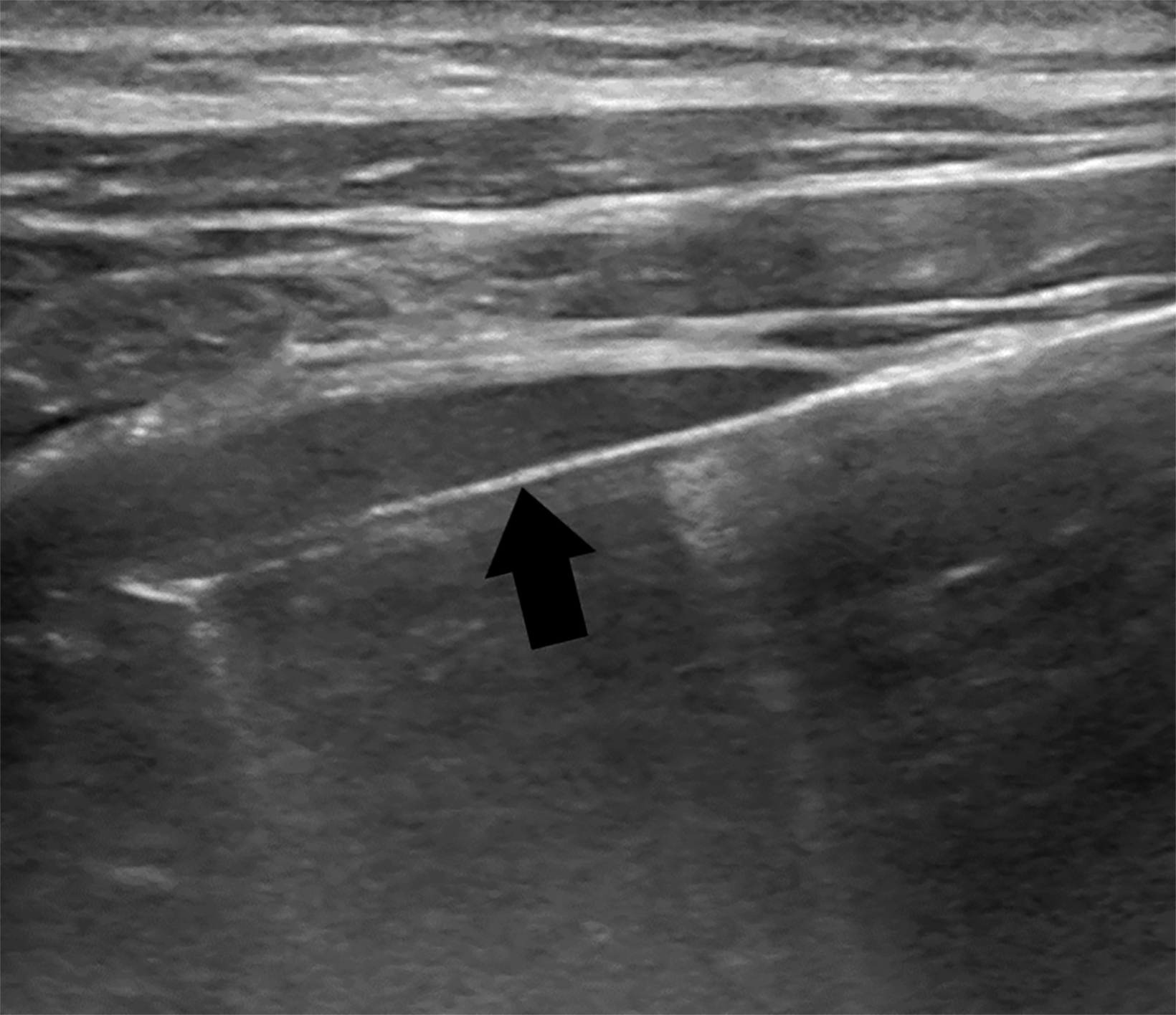
Image-guided PLB has largely replaced the non-image-guided PLB and is usually performed under ultrasound guidance. However, CT and other modalities are also implemented. Image-guided PLB helps to evaluate for the presence of overlying organs and vascular lesions at risk for bleeding, such as hepatic hemangioma (Figure 1).8
The potential complications associated with PLB range from pain and transient hypotension to intraperitoneal or intrahepatic hemorrhage, hemothorax, colon or gallbladder perforation, and pneumothorax. Nevertheless, PLB remains the standard proce- dure for obtaining hepatic tissue specimens.9
Contraindications to Percutaneous Liver Biopsy
Contraindications to PLB include severe congenital and acquired coagulopathy, patient inability to cooperate with positioning and breath-holding, extrahepatic biliary obstruction, and local infection.10 With respect to severe coagulopathy, multiple parameters must be considered when performing a PLB such as international normalized ratio (INR), prothrombin time (PT), platelet count, and non-steroidal anti-inflammatory drug (NSAID) use. In the United States, standard PLB is often withheld in patients with a PT-INR above 1.8, according to Society of Interventional Radiology (SIR) guidelines, but current data reveals uncertainty with regard to the risk of bleeding in such situations. The AASLD position paper on liver biopsy concluded that there is “no specific PT-INR and/or platelet count cutoff at or above which potentially adverse bleeding can be reliably predicted.”10 Hence, the decision to perform liver biopsy in the setting of severe coagulopathy should be made cautiously and weighted according to the risks and benefits of the procedure.10
In addition, patients undergoing PLB must be able to hold their breath to avoid laceration of the liver or liver capsule and subsequent intrahepatic and/or intraperitoneal hemorrhage.9 Extrahepatic biliary obstruction is a relative contraindication, owing to the risk of inadvertent bile leakage; previous studies have shown instances of biliary peritonitis, septicemia, and even patient death.2,11 Use of NSAIDS within 3-5 days of PLB is considered a contraindication, owing to increased risk of bleeding.2 PLB is also contraindicated in the setting of local infection within the pleural space, peritoneal cavity, or right lung, owing to the risk of seeding the liver, resulting in infectious hepatitis or hepatic abscess.10
Relative contraindications to PLB also include morbid obesity, ascites, amyloidosis, and hydatid cysts. Morbid obesity may result in acquisition of an ineffective biopsy specimen owing to the need for deeper needle navigation through subcutaneous fat and tissue to engage the liver. Massive ascites is an absolute contraindication in the utilization of PLB. There is uncertainty as to the increased risk of bleeding with ascites, but the absolute contraindication may stem from risk of inadequate sampling secondary to traversing the ascitic fluid to engage the liver for specimen acquisition.
Biopsies should be performed with caution in vascular tumors or hemangiomas based on the increased risk of bleeding with these tumors. This can be reduced by ensuring there is a healthy liver parenchymal tract towards the targeted lesion.2
Transvenous Liver Biopsy
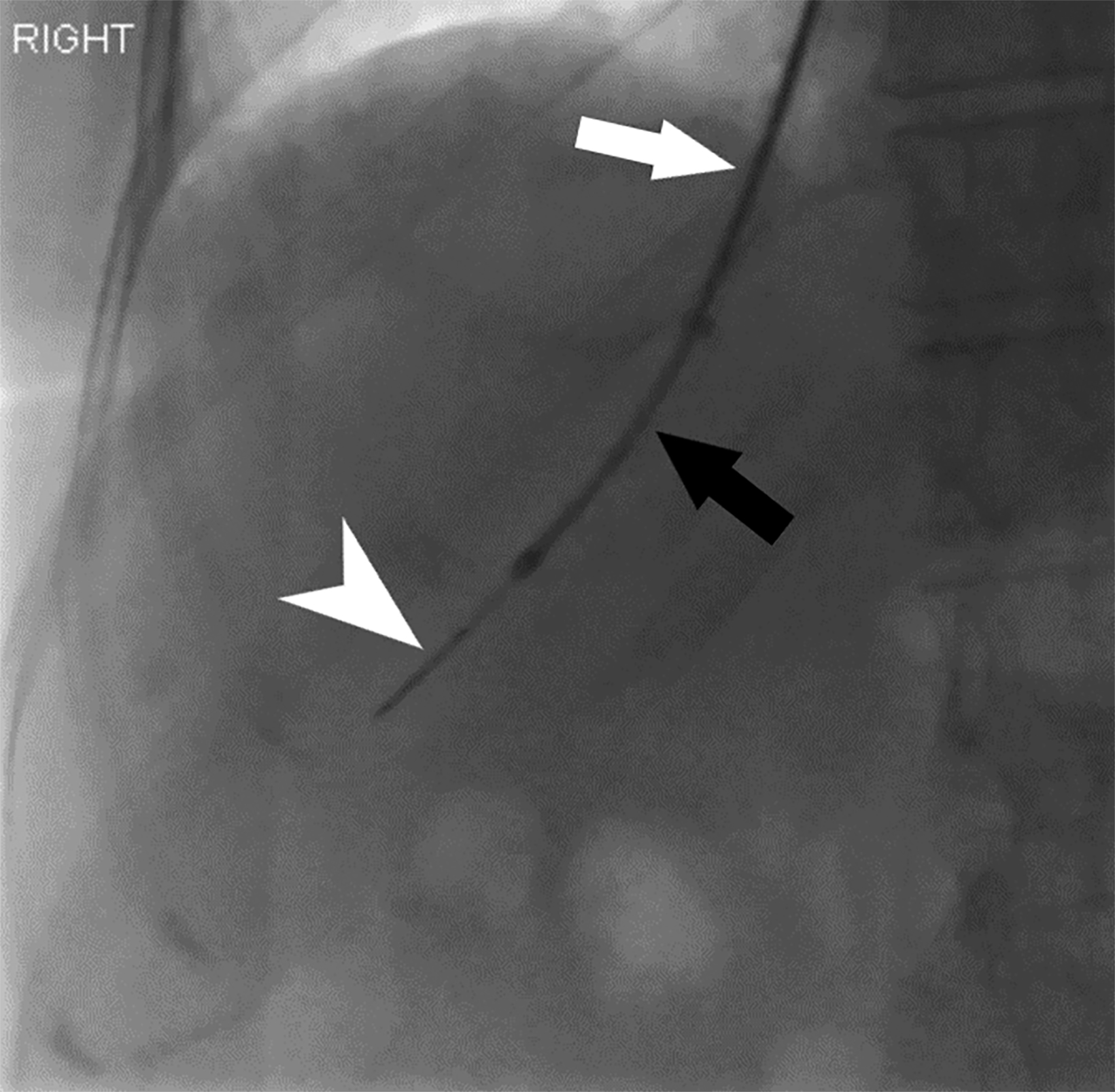
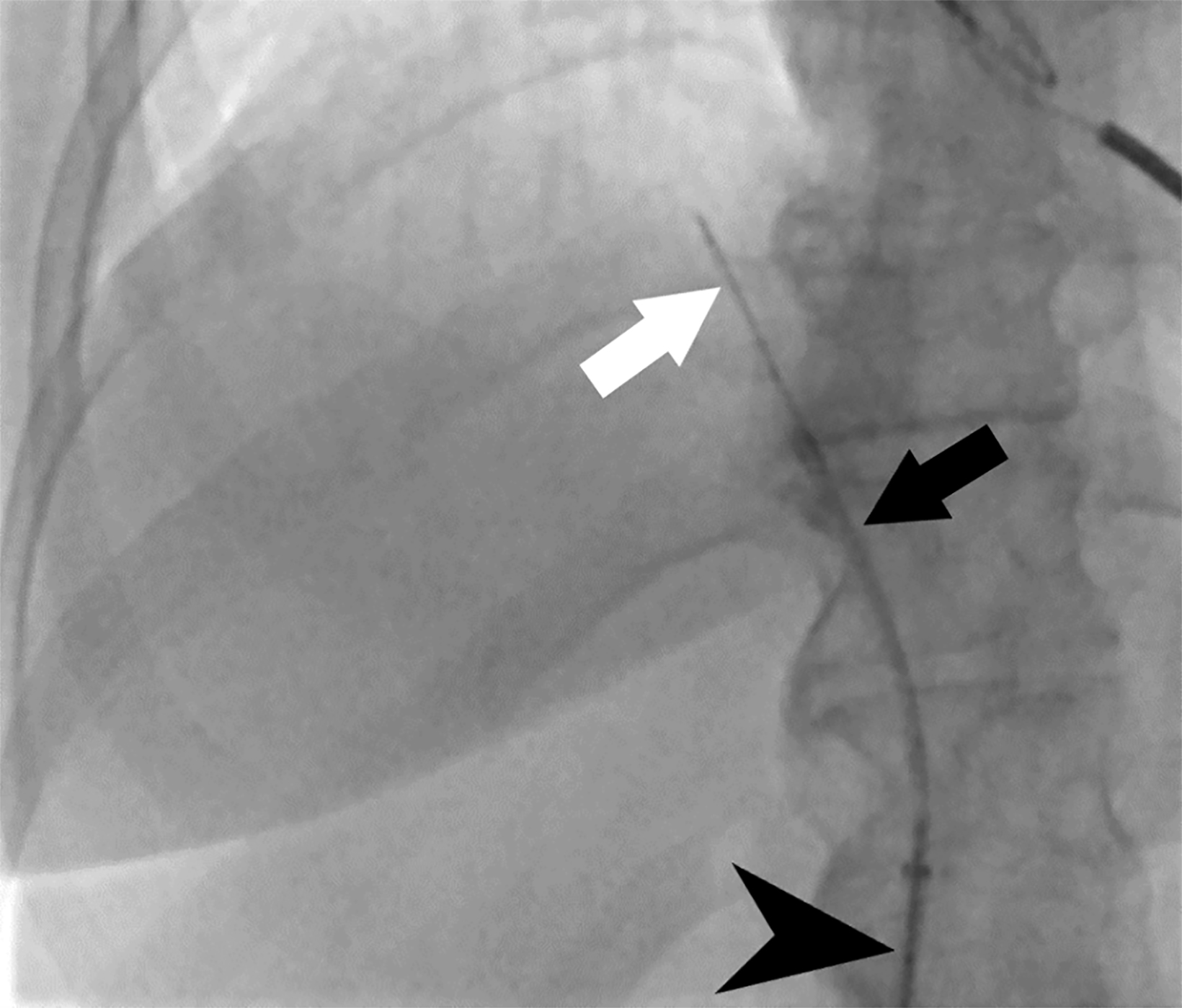
Transvenous liver biopsy (TVLB) is an alternative to PLB and can be effective in patients with contraindications to PLB. Initially described by Dotter in 1964 and clinically performed for the first time by Hanafee in 1967, TVLB is often performed under ultrasound guidance through the right internal jugular vein (IJ), though left IJ and femoral vein access have also been described and practiced safely.12 Following venous access, the right hepatic vein is typically targeted under fluoroscopic guidance.12 Breath-holding can provide additional assistance in selecting the hepatic vein in IJ access, as it will increase the angle between the inferior vena cava and right hepatic vein to above 90°.12 A sampling system such as TLAB (Argon Medical Devices, Plano, Texas), is then advanced over a wire within a guiding sheath to the desired location, ideally the central right hepatic vein, slightly peripheral to the expected location of liver hilum with the needle directed anteriorly (Figure 2). Peripheral puncture should be avoided, particularly in the small, cirrhotic liver, to prevent injuring the liver capsule. Rotational fluoroscopy will confirm the position within the hepatic vein. Middle hepatic vein access is an acceptable alternative, and the sampling direction is typically in a more neutral position in these cases. Operators should also note the differing trajectories, caudal versus cranial, when IJ or femoral access is used, respectively (Figure 3).
Indications for Transvenous Liver Biopsy
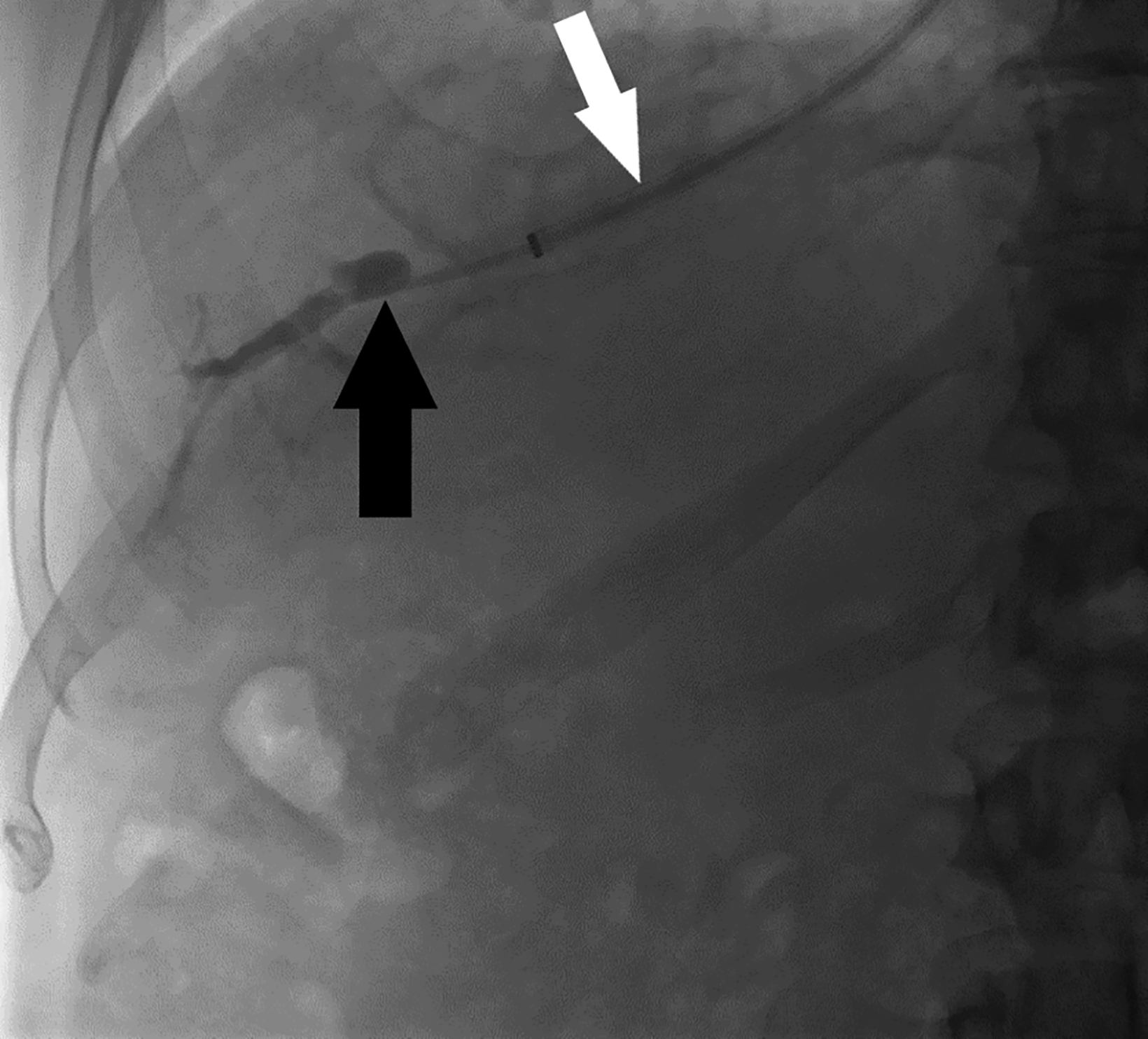
Many indications for TVLB result from contraindications to PLB; these include low PT levels, platelet counts below 50,000/mL, large volume ascites, and use of anticoagulants or antiplatelets that cannot be withheld.12,13 Other indications for TVLB stem from the risk of bleeding associated with conditions such as amyloidosis, chronic renal insufficiency, and hereditary hemorrhagic telangiectasia, among others.12-14 Another advantage to the transvenous approach is its ability to obtain other diagnostic values for assessing suspected portal hypertension. The intravascular catheter position allows for measuring hepatic vein, right atrium, inferior vena cava, and indirect portal pressures (wedge or precapillary pressure) as well as calculating the portosystemic gradient (Figure 4).12
Efficacy and Safety Parameters: Transjugular versus Percutaneous Liver Biopsy
Recently, a retrospective multicenter database review of adult patients undergoing PLB or TJLB sought to identify complication and readmission rates based on demographic and hepatic comorbidity matching.15 The study found that TJLB had a statistically significant lower rate of hematomas compared to that of PLB (0.20% versus 1.20% p= 0.049).15 The same study also identified a higher rate of cardiac complications with the transjugular approach than with the percutaneous approach (0.40% vs. 0.00% p=0.045).15
A modification to standard PLB is the plugged liver biopsy. Plugged liver biopsy involves filling the biopsy needle track with fresh frozen plasma, fibrin sealant, cyanoacrylate glue, or gelatine sponge following removal of the initial coaxial introducer needle.16 A 2010 comparative study of the effectiveness and safety of TJLB versus plugged-PLB (using Gel-foam pledgets) performed in patients with severe liver disease associated with ascites, impaired coagulation, or both found that technical success rates were 97.4% and 99.1% for TJLB and plugged-PLB, respectively.17 TJLB was also shown to have a lower average length of core specimens (1.29 vs 1.43 cm) and lower average number of specimens obtained (2.44 vs 2.8) when compared to those of PLB. However, both methods yielded adequate and sufficient samples for disease diagnosis in that study.17
A 2017 study compared the efficacy of fibrosis staging using TJLB with that of PLB based on a prospective review of patients undergoing 18G TJLB or 16G PLB.18 The subjective interpretation of sample adequacy found a statistically significant difference in the proportion of core fragmentation between TJLB and PLB (35.89% vs 21.67% p = 0.0462), but it also found that a four-core TJLB provided enough tissue volume to meet fibrosis-staging guidelines and approach the effy results of a 16G PLB.18
Conclusion
Transvenous and percutaneous approaches for liver biopsy are important procedures within the interventional radiologist ’s armamentarium. Transjugular liver biopsy provides adequate sampling as compared to that of PLB, with the latter often being considered the primary method for sampling liver tissue. In situations where contraindications exist for PLB, the transjugular approach provides an efficacious route for specimen acquisition in patients with advanced liver disease and hemostatic disor- ders.15 Transvenous liver biopsy also offers utility in manometric evaluation of patients with suspected portal hypertension and for preoperative risk stratification. Interventional radiologists should be familiar with the technical aspects, risks, and benefits of these options.
References
Citation
AHM S, H A, Khaja, Kapoor.Percutaneous and Transvenous Liver Biopsy. Appl Radiol. 2023; (5):8-11.
September 12, 2023