New Chelating Agent Lowers Toxicity in PSMA Radiopharmaceutical Therapy
Images
A newly developed chelator significantly reduces off-target toxicity in PSMA radiopharmaceutical therapy, according to research presented at the 2024 Society of Nuclear Medicine and Molecular Imaging Annual Meeting. By improving the bond between the radioactive metal ion and the PSMA-targeting antibody, the chelator can make PSMA therapy safer and more effective for patients.
Targeted radiopharmaceutical therapy agents require a chelator to bind the radiometal to the cancer-targeting part of the molecule. This ensures that the radiometal does not leak out and cause toxicity to bone marrow, spleen, or the normal clearance organs. In PSMA radiopharmaceutical therapy, there is typically off-target localization in the salivary glands and other tissues. One of the goals of testing the new chelator was to see if toxicity is mitigated by more stably chelating the therapeutic radiometal, in this case Lu-177.
“Reducing off-target toxicity of targeted radiopharmaceutical therapy is essential, especially as PSMA radiopharmaceutical therapy continues to expand,” said Carolyn Anderson, PhD, Simón-Ellebracht Professor in Medicinal Chemistry and professor of Radiology at the University of Missouri in Columbia, Missouri. “A better chelator means that the radiometal accumulates mostly in the tumor, and what does not go to the tumor rapidly clears out of the body. A weaker chelator can cause radiometal to accumulate in off-target locations, leading to slower clearance and contributing to increased toxicity.”
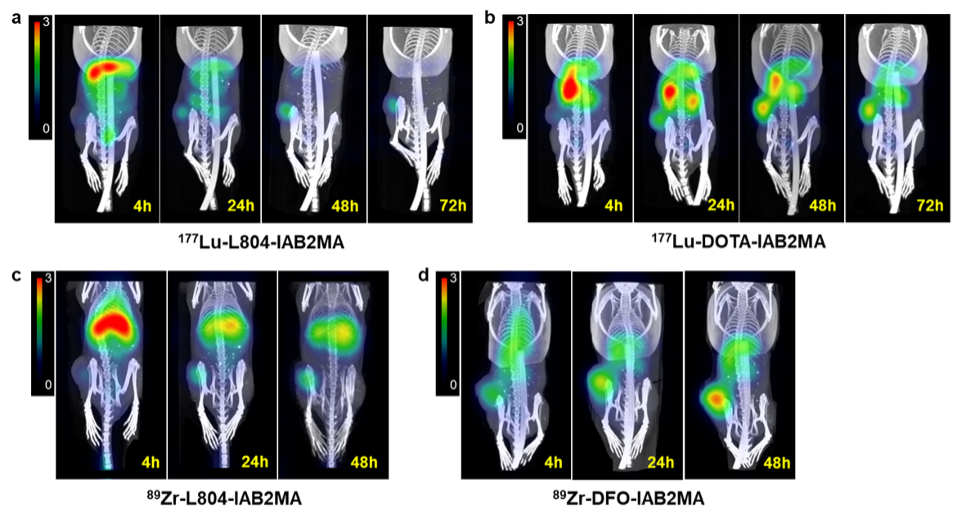
In the study, a newly developed chelator (L804) was attached to the small antibody IAB2MA to create minibody conjugates and radiolabeled with 89Zr and 177Lu (177Lu-L804-IAB2MA and 89Zr-L804-IAB2MA). Similar conjugates were also created using the current gold standard chelators DOTA and DFO (177Lu-DOTA-IAB2MA and 89Zr-DFO-IAB2MA). Preclinical biodistribution, imaging, dosimetry, and efficacy studies were performed in a mouse model of prostate cancer.
Results from in vivo studies showed a significantly lower accumulation of radioactivity in tumor-bearing mice following treatment with 177Lu- and 89Zr-L804-IAB2MA compared to 177Lu-DOTA-IAB2MA and 89Zr-DFO-IAB2MA. Dosimetry analysis indicated significantly lower absorbed doses of 177Lu-L804-IAB2MA in tumor, kidney, liver, and muscle compared to 177Lu-DOTA-IAB2MA. In addition, mice treated with single doses of 177Lu-L804-IAB2MA exhibited significantly prolonged survival and reduced tumor volume compared to unlabeled minibody control.
“The relative merits of stronger chelation are demonstrated here on a well-validated cancer target, using a modified version of a well-studied antibody that has been engineered to be smaller and clear more rapidly from the body,” noted Anderson. “Another advantage of L804-IAB2MA is that it chelates both 89Zr and 177Lu, so only one compound is needed for both radiometals. L804-IAB2MA has strong theranostic potential for 89Zr PET imaging and 177Lu radiopharmaceutical therapy of prostate cancer.”