MRI of benign uterine conditions
Images





Dr. Livermore is a third-year Radiology Resident and, at the time this article was written, Dr. Adusumilli was an Assistant Professor of Radiology, Division of Abdominal Imaging and the Associate Program Director of the Residency Training Program Department of Radiology, University of Michigan Health System, Ann Arbor, MI. † Dr. Adusumilli died March 3, 2007.
Magnetic resonance imaging (MRI) is ideally suited for the evaluation of uterine disease because of its multiplanar imaging capabilities, excellent tissue contrast, and lack of ionizing radiation. This article addresses issues related to benign uterine disease that are relevant to daily practice. We have expanded the discussion beyond the description of typical MRI features of adenomyosis and leiomyomas by presenting diagnostic challenges related to these disease processes. Rarer entities (such as uterine arterio-venous malformations and retained products of conception) are introduced in this article because they have considerable impact on patient management.
Practical issues related to MRI evaluation of adenomyosis
Classic MRI diagnostic criteria
Adenomyosis is characterized by the presence of ectopic endometrial glands in the uterine myometrium with subsequent hyperplasia of the myometrium. 1 The condition usually affects multiparous women who are older than 30 years of age, 2 with a prevalence of 8.8% to 31%. 3-5 Common presenting symptoms include dysmenorrhea, menorrhagia, and abnormal uterine enlargement. 1,6 This clinical presentation poses a diagnostic dilemma, as symptoms are similar in women with uterine leiomyomas. Differentiation from leiomyoma is clinically important, as the treatment of adenomyosis differs from that of fibroid disease. Adenomyosis is typically treated with hysterectomy or, occasionally, with a gonadotropin-releasing hormone (GnRH) analog that can decrease the width of the junctional zone (JZ). 7 A leiomyoma, on the other hand, is potentially treated with uterine artery embolization or uterine-sparing myomectomy. 6
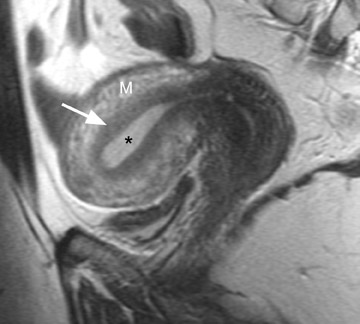
MRI is ideal for differentiating these 2 entities because of its excellent soft tissue contrast. MRI has a high sensitivity (78% to 88%) and specificity (67% to 93%) for the diagnosis of adenomyosis because of its ability to delineate the zonal anatomy of the uterus. 1,8,9 Hricak et al 10 described 3 distinct layers of the uterus that can be identified on T2-weighted (T2W) imaging. The JZ, which is the innermost layer of the myometrium, is of low signal intensity and is located between the endometrium (high signal intensity) and the outer myometrium (intermediate signal intensity) (Figure 1). 11 The JZ is normally <8 mm in maximal thickness.
Adenomyosis can be diffuse or focal and is often associated with enlargement of the uterus, which shows a smooth external contour. 5 Diffuse adenomyosis is diagnosed by the presence of a diffusely thickened JZ that is homogeneously low in signal intensity on T2W imag- 1,12 and isointense to myometrium on T1-weighted (T1W) imaging. 13 The low T2W signal intensity is thought to be secondary to smooth muscle hyperplasia that is incited by the ectopic endometrial tissue. 12 The diagnosis can be made with greater certainty if the JZ exceeds 11 mm in thickness (Figure 2) and is equivocal when the JZ measures between 8 and 11 mm. 1,8
One must be careful in distinguishing between adenomyosis and normal menstrual changes. These changes are most pronounced early in the menstrual cycle (days 1 and 2) and may cause significant thickening of the JZ to >12 mm, thus causing a false-positive diagnosis of adenomyosis. 9 Characteristic imaging findings that increase diagnostic confidence and improve specificity for the diagnosis of adenomyosis include high signal intensity foci in the JZ on T1W and T2W imaging that represent ectopic endometrial tissue, endometrial cysts, or small foci of hemorrhage (Figure 2). 1,2,5,8
Uterine mass: Adenomyoma versus leiomyoma
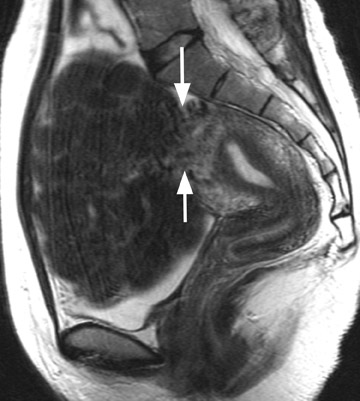
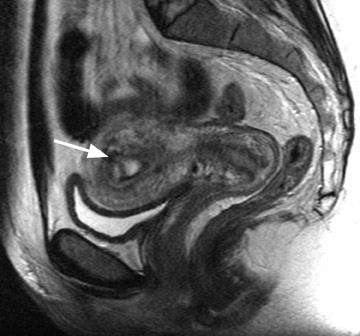
Focal adenomyosis classically appears as a low-signal-intensity mass in the myometrium with ill-defined borders on T2W imaging 1,13 and isointensity to the surrounding myometrium on T1W imaging. 5 These ill-defined masses commonly contain small foci of high T1W and/or T2W signal intensity and do not exhibit a pseudocapsule. 5
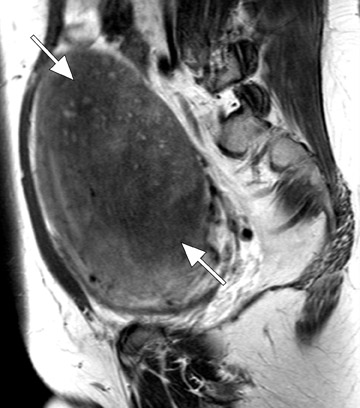
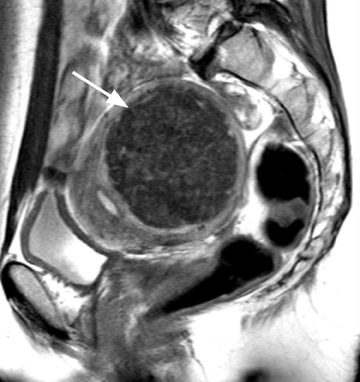
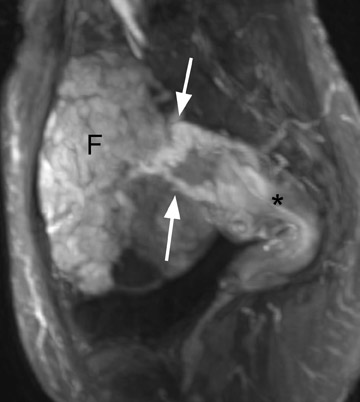
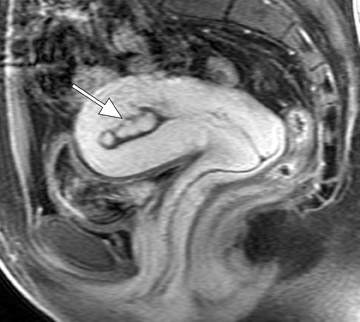
Diagnostic problems arise when one must differentiate focal adenomyosis from a leiomyoma. MRI is quite accurate in diagnosing a leiomyoma with a sensitivity of 88% to 93% and a specificity of 66% to 91% a,5,8 and in differentiating leiomyoma from focal adenomyosis. Togashi et al 2 correctly diagnosed 92 of 93 cases as either leiomyoma or adenomyosis in women with an abnormally enlarged uterus. A typical feature of an adenomyoma is a poorly marginated mass with ill-defined borders that causes relatively little mass effect (Figure 3). On the contrary, a leiomyoma has a well-defined border and exerts a greater degree of myometrial mass effect and distortion (Figure 4). 2,5,14 Leiomyomas usually compress the adjacent myometrium, creating the appearance of a pseudocapsule, which is not seen with adenomyosis, as the ectopic endometrial glands interdigitate with the normal smooth muscle of the myometrium. 13
Mark et al 13 also found that leiomyomas were typically round in shape, whereas adenomyomas were most often oval with their long axis paralleling the uterus. Another differentiating characteristic is the presence of adjacent dilated vessels at the interface with the myometrium, which is commonly associated with leiomyomas and is not seen with adenomyosis. Mark et al 13 found that 42% of leiomyomas had such dilated vessels, whereas Togashi et al 6 found no cases of adenomyosis with dilated vessels. An additional imaging pitfall is the incorrect diagnosis of a focal myometrial contraction as either an adenomyoma or a leiomyoma. A uterine contraction can appear as a myometrial mass that has low signal intensity on T2W imaging and that bulges into the endometrial cavity. The low signal intensity on T2W imaging is secondary to decreased water content in the area of contracted myometrium. 9 The distinction can be easily made, as a myometrial contraction is, by definition, a transient phenomenon that should resolve on subsequent sequences. 12
Practical issues related to MRI evaluation of uterine fibroids
Uterine leiomyoma: Classic MRI features
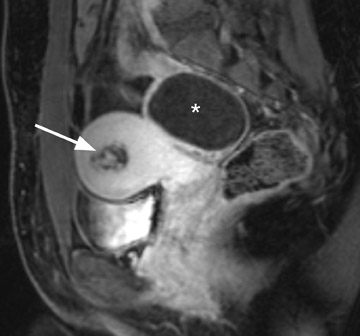
Uterine fibroids are composed of a combination of smooth muscle cells and fibrous connective tissue and can be found in approximately 20% to 30% of reproductive age females. 12 As these masses enlarge, they commonly outgrow their blood supply and undergo varying degrees of necrosis, which accounts for their variable signal intensities on MRI. Typically, leiomyomas are low in signal intensity relative to their surrounding myometrium on T2W imaging (Figure 4) and are isointense to myometrium on T1W imaging. 11,15 These signal characteristics are related to the most common form of degeneration (60%), which is hyalinization throughout the leiomyoma. 12,15 Weinreb et al 16 defined diagnostic criteria for a leiomyoma to include a uterine mass that is predominantly hypointense compared to myometrium on T2W imaging and predominantly hypointense on T1W imaging.
Solid pelvic mass: Pedunculated uterine fibroid versus adnexal mass
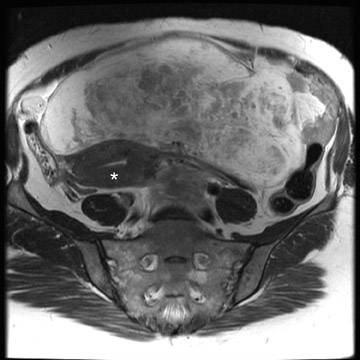
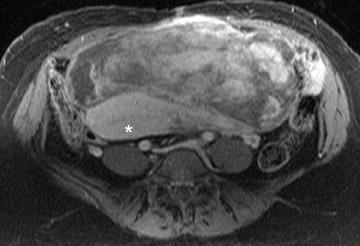
Leiomyomas can be classified by their location in the uterus as submucosal, intramural, or subserosal. In some instances, it can be difficult to differentiate a large exophytic, subserosal leiomyoma from a solid adnexal mass such as an ovarian neo- plasm. Differentiation is clinically very important because of differences in treatment and prognostic implications. MRI in combination with the patient's clinical findings can be invaluable in making this distinction and can avoid unnecessary laparoscopy and/or exploratory surgery. Location is an important distinguishing characteristic. If the mass can be definitively separated from the ovaries or is contiguous with the round ligament, than an ovarian etiology is unlikely. 17
A well-described MRI feature that is helpful in the evaluation of large pelvic masses has been referred to as the "bridging vascular sign," which consists of vessels and/or signal voids that extend from the uterus to supply a pelvic mass. 17 The identification of the bridging vascular sign increases the diagnostic confidence that a large pelvic mass is a uterine leiomyoma. The bridging vessels can be identified as enhancing tubular structures on contrast-enhanced T1W imaging or as flow voids on a T2W fast spin-echo sequence (Figure 5). In a study by Kim et al, 17 the bridging vascular sign was present in 20 of 26 exophytic leiomyomas and was absent in all other adnexal masses, resulting in a diagnostic accuracy of 80%.
Uterine leiomyoma: Degeneration versus malignant transformation
Another diagnostic dilemma encountered on MRI is the differentiation of a degenerating fibroid from malignant transformation of a fibroid into a leiomyosarcoma. Sarcomatous transformation of a pre-existing leiomyoma is thought to be rare. It is believed that most leiomyosarcomas actually arise independently from the myometrial smooth muscle cells. The clinical significance of this differentiation is important, in light of the growing use of uterine-sparing leiomyoma treatment such as myomectomy, GnRH analog, and uterine artery embolization (UAE). 18 Clinically, malignancy can be suspected if there is a rapidly enlarging pelvic mass, if a mass does not involute after menopause, or if a mass does not decrease in size following GnRH therapy. Unfortunately, the diagnosis of sarcomatous degeneration is most commonly made as an incidental pathologic diagnosis in 0.5% of resected leiomyomas. 16,19 Suspicious imaging findings of malignant degeneration on pelvic MRI include ascites, lymph node enlargement, and peritoneal seeding. However, earlier detection is obviously desired.
Initially, investigators thought that rapid growth of a leiomyoma over time would be an accurate predictor of sarcomatous degeneration. However, in a retrospective review of 580 leiomyosarcomas, <3% of sarcomas were associated with a rapidly enlarging uterus. Additionally, in the same study, only1 of 371 women who underwent resection for a rapidly growing leiomyoma was found to have a leiomyosarcoma. 12 Therefore, rapid growth is now believed to be an unreliable indicator of malignant transformation. The same investigators proposed that a better indicator for predicting malignant transformation was an irregular margin of the uterine leiomyoma on MRI. 12 Pattani et al 20 concluded that malignant potential should be considered whenever a degenerating fibroid has an ill-defined contour. Utilizing an ill-defined margin as their criteria for diagnosing malignant potential, Schwartz et al 21 achieved a 100% preoperative accuracy in diagnosing 4 of 4 leiomyosarcomas; they also did not incorrectly label benign fibroids as malignant. However, the specificity of this finding has not been established. 20
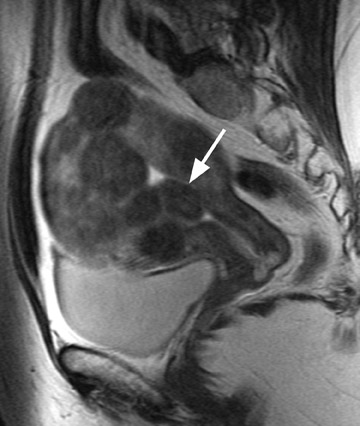
A small study of 12 patients with known malignant tumors performed by Tanaka et al 18 found that high signal intensity on T2W imaging in >50% of a uterine mass was predictive of malignancy. On T2W imaging, high signal intensity due to normal cellular degeneration does not typically involve >50% of a fibroid, although overlap can be seen between both entities (Figure 6). Similarly, the presence of small areas of nonenhancing high signal intensity on T1W imaging was also found to be predictive. 18 When a mass is predominantly hyperintense on T2W imaging, one must be careful not to dismiss the lesion as a simple leiomyoma, as this signal alteration can be due to malignancy or sarcomatous degeneration. 15
Role of MRI in the evaluation of women undergoing uterine artery embolization
Uterine artery embolization is a safe and effective option for the treatment of symptomatic leiomyomas. Common symptoms that often require treatment include pain, bulk symptoms, and abnormal uterine bleeding. Uterine artery embolization is an option when uterine preservation is desired or when the patient is unable to tolerate an open surgical procedure. 22
Appropriate patient selection is important when planning UAE to avoid serious complications. Absolute contraindications include ongoing pregnancy, active infection, and uterine or adnexal malignancy. Relative contraindications based on imaging studies are continually evolving but have traditionally included pedunculated fibroids (subserosal or submucosal) that are thought to be at increased risk for detachment from the uterus postembolization. 22,23 Detached subserosal fibroids can cause intraperitoneal adhesions and chronic peritonitis, whereas detached submucosal leiomyomas can undergo transcervical expulsion with prolonged recovery and sometimes require additional surgery (Figure 7). 22
Previously, leiomyomas >10 cm in dimension were considered a relative contraindication for UAE. For instance, Pelage et al 24 stated that UAE should not be performed for fibroids >10 cm in diameter because of predisposition to complications such as infection and uterine injury. Additionally, large leiomyomas are more likely to have a collateral blood supply that is separate from the uterine arteries, making UAE less effective with a smaller volume reduction postembolization. 22 However, subsquent studies performed by Katsumori et al 25 reported no increased risk to patients who underwent UAE for fibroids on the basis of tumor size. They found no difference in postprocedural pain, recovery, or leiomyoma size reduction at 4 months and 1 year postprocedure. 25
MRI is an excellent modality for the evaluation of potential UAE patients. It is superior to ultrasound based on its larger field of view, better spatial resolution, and ability to detect concomitant pelvic processes. 26 MRI is accurate in localizing a leiomyoma as subserosal, intramural, or submucosal and in the identification of a thin stalk. Pedunculated, subserosal fibroids have previously been defined as having a stalk that is <50% the diameter of the actual leiomyoma. 23 This is considered a contraindication to UAE. However, Katsumori et al 23 studied 196 women, 12 of whom had pedunculated subserosal fibroids with a mean stalk diameter of 3.1 cm (2.0 to 5.5 cm). Patients whose fibroid had a stalk <2 cm were excluded from undergoing UAE. The final study group had no complications, such as detachment of the leiomyoma from the uterus or other permanent adverse sequela. 23 Patients were followed at both 4 months and 1 year postembolization, and the leiomyoma stalk diameter was found to be stable, whereas the leiomyoma had decreased in size. Therefore, it was concluded that UAE was safe for pedunculated, subserosal leiomyomas with stalk diameters >2 cm. 23
Another imaging feature that is important to assess on pre-UAE MRI is the presence of enhancement within a leiomyoma. The degree of enhancement correlates with the vascularity of the mass and suggests a better response after embolization (Figure 8). 22 Nikolaidis et al 26 studied 94 women and found that 20% of women had nonenhancing leiomyomas on their preprocedural MRI that were considered nonviable for UAE. However, in two thirds of the women, the nonviable leiomyoma was not the dominant fibroid. In only 6% of the women was the nonviable, avascular leiomyoma also the dominant fibroid, in which case UAE would not be expected to reduce blood flow or result in a favorable response. 26
Some studies have shown that cellular leiomyomas, with high signal intensity on T2W imaging, exhibit a greater percentage of volume reduction postembolization. In contrast, degenerating leiomyomas with high signal intensity on T1W imaging are associated with a smaller volume reduction postembolization. 22,27,28 Location can also be predictive of successful postembolization volume reduction. Smaller leiomyomas in a submucosal location are more likely to have the greatest degree of volume reduction. 27 Studies have shown that a submucosal location is strongly predictive of outcome with a 30% to 40% greater volume reduction following embolization compared with either intramural or subserosal locations. 26,27,29
Role of MRI in diagnosis of postpartum and postabortion uterine abnormalities
Uterine arteriovenous malformation
A uterine arteriovenous malformation (AVM), which is classified as either a congenital or an acquired lesion, is composed of a tangle of variously sized arteries and veins without histologic evidence of an intervening capillary bed. 30 Uterine AVMs are most commonly seen in women who are 20- to 40-years-old and can result in symptoms of menorrhagia or menometrorrhagia. 31 Typically, an AVM is acquired and secondary to trauma related to spontaneous or therapeutic abortion, endometrial carcinoma, or gestational trophoblastic disease. 30,32 There is also a greater potential for the formation of abnormal arteriovenous connections following hysterectomy and cesarean section. 31
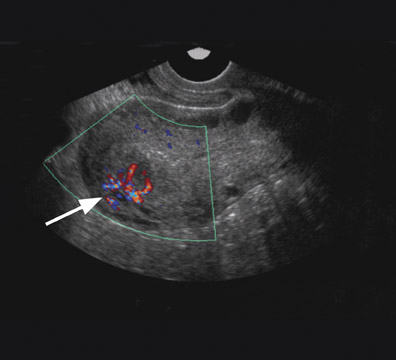
Uterine AVMs have nonspecific and often confusing imaging features on gray-scale sonography, including multiple tubular anechoic spaces within the myometrium. 30 Color Doppler is valuable and can show marked hypervascularity of the mass (Figure 9) as well as reversal of flow. 30,32 Spectral Doppler reveals low-resistance high-velocity arterial flow (resistive index of 0.25 to 0.55), and low pulsatility (pulsatility index 0.40 to 0.59). Sonography may detect a vascular abnormality in the myometrium and offer a potential diagnosis of AVM. However, arteriovenous shunting, in itself, is a nonspecific finding that can occur in other conditions such as pregnancies, missed abortions, trophoblastic disease, and ectopic pregnancies. MRI is able to confirm the diagnosis, better define the extent of the lesion, 30 and offer a more accurate characterization in the setting of equivocal sonographic findings.
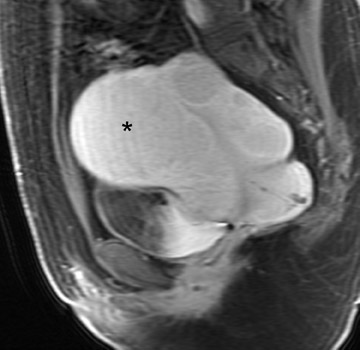
Typical MRI findings of an AVM include a focal uterine mass that consists of a group of distinct, serpiginous flow voids on T2W imaging with an ill-defined border that disrupts the JZ and is associated with prominent parametrial vessels (Figure 9). 30,32 Dynamic contrast-enhanced T1W gradient-echo imaging will also reveal opacification of the vessels within the uterus and adjacent parametrium as well as depict an early draining vein. An AVM has a propensity to erode into the endometrial cavity if it reaches a large enough size (Figure 9).
The diagnosis of uterine AVM on MRI has important therapeutic implications because it 1) may prevent dilatation and curettage that could otherwise result in catastrophic bleeding and 2) may lead to catheter angiography that can both diagnose and treat the lesion with embolization, thereby avoiding more invasive treatment such as hysterectomy. 31 Angiography reveals abnormal stasis of contrast within a tangle of vessels that are supplied by enlarged feeding arteries and have early venous drainage during the arterial phase of image acquisition. 30 Management of a patient with a symptomatic AVM also includes control of vaginal bleeding with uterine packing, maintenance of hemodynamic stability with blood transfusions, and administration of medications (such as 15-methyl prostaglandin F2 alpha and parenteral estrogen and progestin). 31
Stable patients without heavy bleeding can be treated conservatively and followed expectantly.
Retained products of conception (placental polyps)
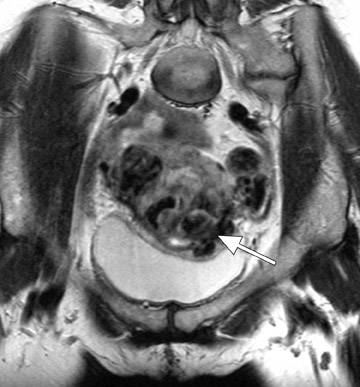
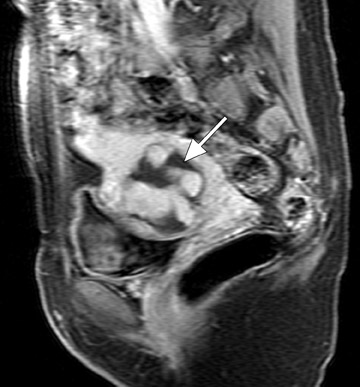
Another vascular-appearing uterine abnormality that is well-suited to MRI evaluation is retained products of conception (RPOC)-the retention of placental tissue within the uterine cavity after delivery or abortion. The placental fragment may eventually form a placental polyp that can be retained long after the initial pregnancy. Retained products of conception have been reported to complicate approximately 1% of all pregnancies 33 and commonly present with vaginal bleeding either late in the postpartum period or even months to years after the pregnancy. The diagnosis may be evident when considering the patient's clinical presentation and laboratory data (β-HCG). 32 Patients with postpartum hemorrhage are initially evaluated with ultrasound, which reveals echogenic material within the endometrial cavity. 32 However, occasionally, RPOC may appear as an intramural mass (consisting of serpiginous vessels) that shows vascularity on color Doppler and thus mimics a uterine AVM (Figure 10). It is important to make the distinction between RPOC and AVM, as RPOC are routinely treated with dilation and curettage that could result in hemorrhage in the presence of a uterine AVM. 32 In cases of confusing sonographic findings, MRI is the ideal next step for identifying imaging features that would suggest an AVM and also for delineating the exact location of the lesion.
MRI findings of a classic RPOC include a soft tissue mass within the endometrial cavity that shows heterogeneous signal intensity on T1W and T2W imaging and avid enhancement on gadolinium-enhanced T1W images (Figure 10). The mass usually disrupts the JZ and causes some degree of myometrial thinning. While the lesion can show avid enhancement (Figure 11), it is less likely to be associated with an early draining vein and an increased number and size of parametrial vessels. 33 However, there has been a case report of an RPOC showing serpiginous signal voids on T1W imaging and irregular enhancement in the uterine wall, thereby masquerading as a uterine AVM. 32 No enhancing soft tissue mass was identified in the endometrial cavity to confidently diagnose retained placental tissue. Although rare, this case illustrates the overlap of uterine AVM and the atypical form of aged RPOC that presents as an intramural mass with prominent arteriovenous fistulas secondary to necrosis of chorionic villi in the placenta. 32
Gestational trophoblastic disease (GTD) or placental site trophoblastic site disease (PSTD) can also have very similar MRI features to that of RPOC. Again, this distinction is extremely important because of differences in treatment, with trophoblastic disease requiring chemotherapy. Prior studies have suggested that RPOC can be distinguished from GTD based on the lack of enhancement of RPOC and brisk enhancement of GTD. 34 However, a small series of RPOC described by Noonan et al 33 found this sign to be unreliable, as RPOC was shown to enhance in every case. Therefore, the differentiation may not always be made solely based on imaging findings and requires correlation with serial β-HCG levels. While the β-HCG level remains elevated to 1000 mIU/mL or more in the setting of GTD, it generally decreases to zero or near zero with RPOC and should not be elevated with uterine AVMs. 30,33
Conclusion
MRI is an invaluable problem-solving tool when evaluating women with benign uterine conditions. The use of T2W and gadolinium-enhanced T1W imaging is particularly important in accurately localizing and characterizing uterine masses. An awareness of important imaging principles related to specific uterine diseases, as discussed in this article, should help the practicing radiologist solve these radiologic dilemmas.
Acknowledgment
Dr. Livermore and the editors of this journal would like to thank Hero K. Hussain, MD (Assistant Professor of Radiology, Director of Clinical MR Service, and Chief, Body MRI, Department of Radiology, University of Michigan Health Service, Ann Arbor, MI) for her assistance in reviewing this article following Dr. Adusumilli's death.
Related Articles
Citation
. MRI of benign uterine conditions. Appl Radiol.
September 12, 2007