Minimizing the Pain of Local Anesthetic Injection
Images
Minimizing pain is an important component of any procedure. When poorly controlled, procedural pain can be an important source of patient dissatisfaction.1 While intravenous sedation decreases patient pain and anxiety, administering a local anesthetic is critical to generate as little pain as possible. While they are excellent at decreasing pain, local anesthetics can cause pain when infused. Patients are often cautioned about the “stick and burn” that accompanies injection. However, when the injection is performed properly, pain can be limited to the initial needlestick and followed by an otherwise pain-free procedure.2,3 With the number of cases performed in an average interventional radiology (IR) practice daily, there are many opportunities to refine local anesthetic technique and improve patient satisfaction.
Mechanism of Pain
Nociceptors are free nerve endings that originate from peripheral nerves and are sensitive to noxious mechanical, temperature, or chemical insults.2,4,5 There are two types of nociceptive axons, A-delta fibers and C-fibers.2,4,5 A-delta fibers are myelinated fibers responsible for communicating sharp, transient pain. C-fibers are unmyelinated and communicate dull pain. When exposed to inflammatory signaling molecules, the depolarization threshold of peripheral nociceptors is lowered. Pain signals are carried to the dorsal horn of the spinal cord and ascend centrally through the spinothalamic and spinoreticulothalamic tracts. The spinothalamic tract communicates sharp pain, and the spinoreticulothalamic tract communicates dull, poorly localized pain.
Pain from local anesthetic injection comes from two sources.2 The first is mechanical trauma from needle insertion, which is carried by A-delta fibers. The second is from infiltration, which distends the affected tissues and exposes nociceptors to the acidic solution. This component is carried by C-fibers.
Lidocaine
Lidocaine is near ubiquitous in surgical and interventional suites, owing to its excellent safety profile.6 This paper will focus on the use of lidocaine, although the principles translate to other local anesthetics.
When injected, lidocaine diffuses through the cell membrane and blocks the voltage-gated sodium channels in peripheral nociceptors.1,2 Onset of analgesia is usually under one minute and can last 2-5 hours.6,7,8 In adults, the maximum dose of lidocaine is 4 mg/kg (total dose no greater than 350 mg) and 7 mg/kg (total dose no greater than 500 mg) when combined with epinephrine.6,7 In children, the maximum dose of lidocaine is 2 mg/kg (total dose no greater than 150 mg) and 4.5 mg/kg (total dose no greater than 150 mg) when combined with epinephrine.6
Procedural Preparation
Buffering with Sodium Bicarbonate
The physiologic pH of the body is slightly alkaline, ranging between 7.35 and 7.45. In contrast, lidocaine has a pH of 6.09 in the 1% formulation and 6.00 in the 2% formulation.9 With the addition of 1:100,000 epinephrine, which will be discussed elsewhere, the pH drops to 4.2, which is about 1000 times more acidic than physiologic pH.9 The acidic nature of lidocaine is theorized to be one source of pain with local anesthesia.9 This can be addressed by buffering lidocaine with sodium bicarbonate to achieve a neutral pH. At a physiologic pH, more molecules of lidocaine diffuse through nociceptive cell membranes resulting in a faster onset of action.10 Several double-blind, randomized controlled studies (RCT) have confirmed a statistically significant decrease in pain when buffered lidocaine is used.11,12
The most widely reported buffering technique is the addition of 1 mL of 8.4% sodium bicarbonate to 10 mL of 1% lidocaine with 1:100,000 epinephrine.9 The resulting 1:10 mixture has a pH of 7.4. This cocktail is stable at room temperature for at least one week and has no risk of precipitation.9 The concentration of epinephrine can be expected to fall by 25% per week, although this can be obviated by creating the mixture at the beginning of the day or prior to every case.2
Addition of Epinephrine
Lidocaine most commonly comes in 1% and 2% formulations, which can be modified with the addition of epinephrine. Classically, lidocaine with epinephrine is used to reduce procedural bleeding. Vasoconstriction from the added epinephrine is short lived but has the benefit of blanching the skin, which can help identify areas that have already been anesthetized. In addition, vasoconstriction reduces blood flow to the treatment zone, increasing local drug concentration.2 Epinephrine also results in faster onset of pain relief and increases the duration of relief up to 10 hours.13
Warming to Body Temperature
The solubility of lidocaine increases with rising temperature.14 Like buffering with bicarbonate, more lidocaine diffusing across nociceptive cell membranes results in a faster onset of action.15,16 Lidocaine can be warmed to 70° C without affecting its efficacy.15 A 2017 RCT showed a statistically significant decrease in pain with warming.17 Pain relief with warming is independent of the pain relief from buffering to a neutral pH.16 In addition, buffering and warming have a synergistic effect, and provide the greatest relief when combined.18,19 Finally, warming lidocaine has no detrimental effect on the duration of analgesia.16
Use of Finer Needles
Higher-gauge needles require less force to pierce the skin, contact fewer nociceptors, and slow the speed of injection. Several studies have investigated the use of higher gauge needles (23 to 32 gauge) and found a linear decrease in pain with increasing needle gauge.20-23 As a result, 27–32-gauge needles are recommended.
Maintaining needle sharpness is also important for pain control. A blunt needle requires more force to pierce the skin, increases friction as the needle passes through tissue, and generates more pain.21 Needle sharpness can be maintained by minimizing the number of needle insertions, exchanging blunted needles for new ones, and using separate needles for drawing up and injecting lidocaine.21,24
Patient Preparation
Skin Preparation
Applying a eutectic mixture of local anesthetics (EMLA) cream can help reduce the pain of the needle transgressing the skin. The most common EMLA cream is a topical oil and water emulsion of 2.5% prilocaine and 2.5% lidocaine. Several studies have shown a statistically significant decrease in pain when the skin is pretreated with a topical anesthetic.2,25 The primary limitations of EMLA cream are the time to maximum effect and the depth of penetration. EMLA requires at least 60 minutes to anesthetize to a depth of 3 mm and up to 120 minutes to a depth > 5 mm.25,26
An alternative to EMLA cream is cooling the skin,27 usually with an ice pack. This technique originated in dentistry, as topical anesthetics cannot be used on mucosal surfaces. Cooling is less efficacious than topical anesthetics but has a faster onset of action and is more cost-effective.27
Management of Expectations and Distraction
Anxiety plays a prominent role in pain perception, and many patients experience anxiety prior to procedures.28,29 Speaking with patients and establishing a level of trust can set the tone for the remainder of the encounter. Distraction can also help decrease pain. Engaging the patient in conversation; asking them to perform a task and/or slowly exhale during injection; nurse coaching; and hypnosis can all decrease pain.30 Finally, asking the patient to look away from the injection has been shown to decrease pain perception.31
Injection Technique
Gate Theory
When a nociceptor is activated, the pain signal is transmitted to first-order neurons at the synapse. When pain is the only signal transmitted, the strength of the signal is maximized. However, when nearby non-pain-signaling axons are also activated, transmission of the pain signal at the synapse is decreased.32 In simple terms, there is a limit to the amount of pain that can be communi- cated at the synapse, and this can be diluted by additional stimuli. In practice, application of light touch, pressure, or vibration near the site of injection can decrease pain.33,34 An additional technique is to spray a small amount of lidocaine on the skin immediately prior to needle insertion. This simple technique has shown a statistically significant decrease in pain.35
Needle Insertion
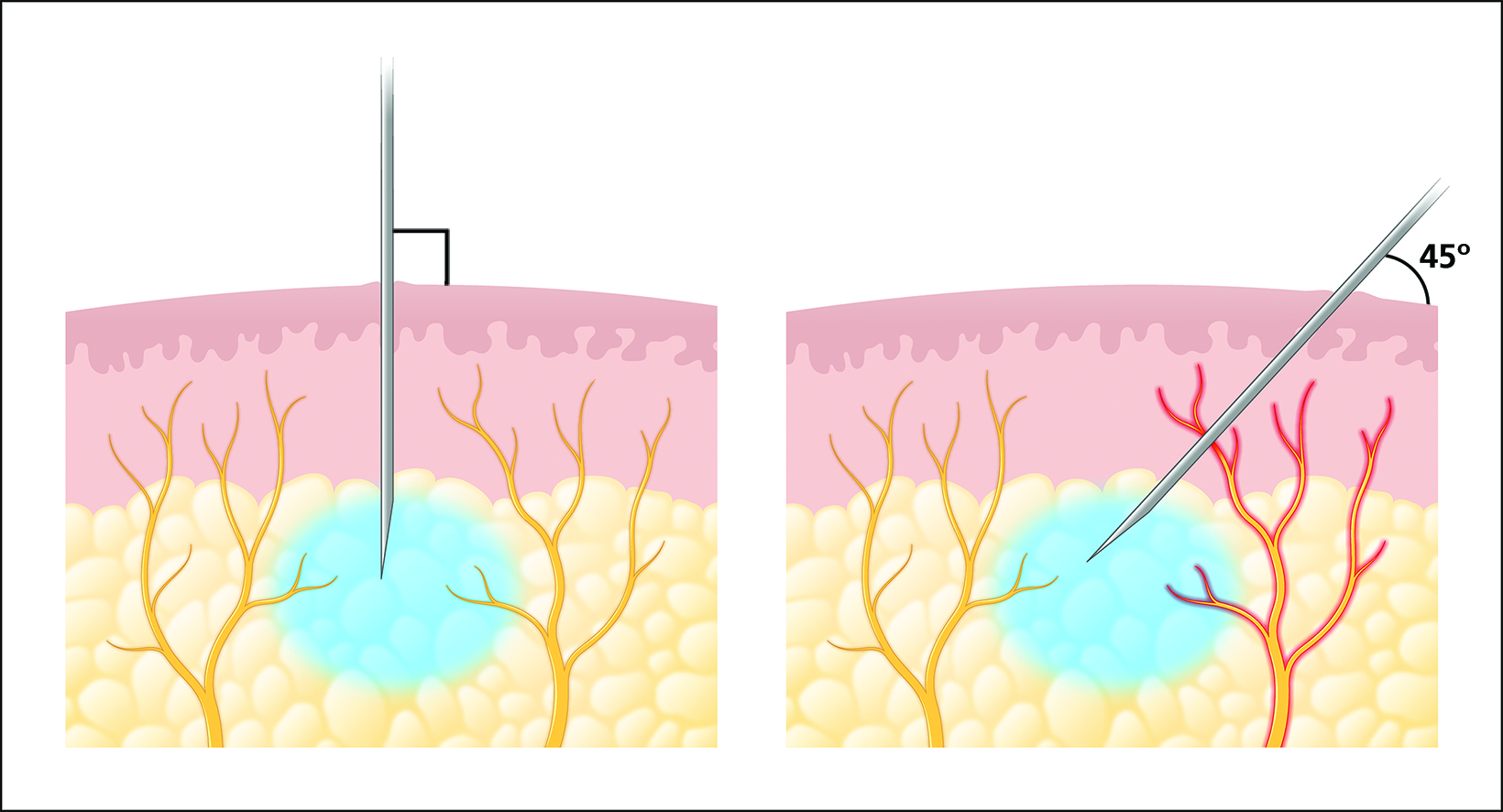
When the needle enters the skin, it directly stimulates nociceptors, which transmit sharp pain through A-delta fibers. The needle should be quickly inserted into the skin perpendicular to the skin surface (Figure 1). Theoretically, this minimizes direct contact with nociceptors. This technique has been shown to decrease pain when compared to inserting the needle at a 45-degree angle.36 Once inserted, care should be taken to keep the needle as still as possible, which will also decrease pain.37 The number of needle sticks should also be minimized.
Site and Rate of Injection
Once the needle is inserted, lidocaine should be injected subcutaneously rather than intradermally. Subcutaneous injection generates less pain than intradermal injection while achieving the same analgesic effect.2 A deeper injection also treats the nerve roots supplying the dermis, making intradermal injection unnecessary.2 When a wider region must be anesthetized, lidocaine should be injected while advancing the needle to anesthetize nociceptors prior to needle traversal.
The rate of injection is also critical in decreasing pain.2,3 Several studies have demonstrated less pain with slower versus faster injections.38,39 Determining how slowly to inject, however, is difficult. The two studies cited judged a slow injection as one ranging between 2 and 4mL/min and a fast injection ranging between 7.5 and 12 mL/min. In the authors’ experience, an injection rate of 2-4 mL/min is usually sufficient.
Physician Attention and Care
Finally, as lidocaine is infused, the physician should closely watch the patient for signs of pain, paying special attention to the face. Subtle signs such as wincing, pursing of the lips, and clenching the jaw can give immediate feedback that pain is being generated. With time, practice, and close attention to the patient, physicians can refine their technique and approach mastery of injection.
Anesthetic Use in Interventional Radiology
Local anesthesia is used in almost every procedure in IR, and these preparation and techniques are sufficient in many cases. However, they may be modified in certain situations to attain the best results. The most important of these circumstances are any interventions that transgress the pleura, peritoneum, or periosteum, and those that traverse a solid organ.
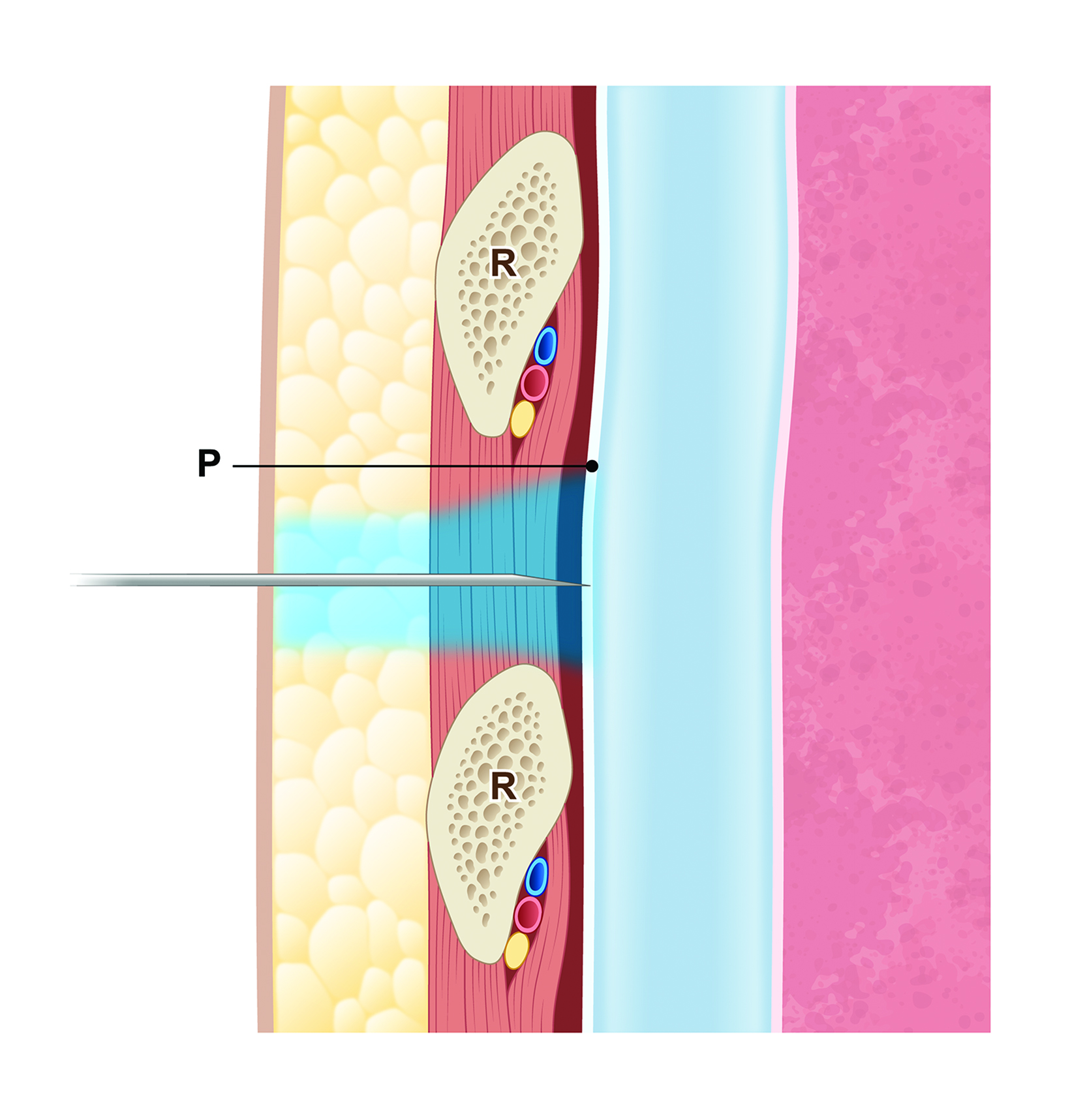
The parietal pleura and peritoneum are well-innervated and transgression with a needle will result in sharp, well-localized pain. Special care should be taken to anesthetize these layers, otherwise the patient will still feel sharp pain as the needle passes through (Figure 2). On ultrasound, the pleural or peritoneal linings appear as a thin, echogenic layer. When lidocaine is infused liberally in and around the lining, the echogenic line will disappear. The anesthetized area can then be transgressed by the relevant device without discomfort.
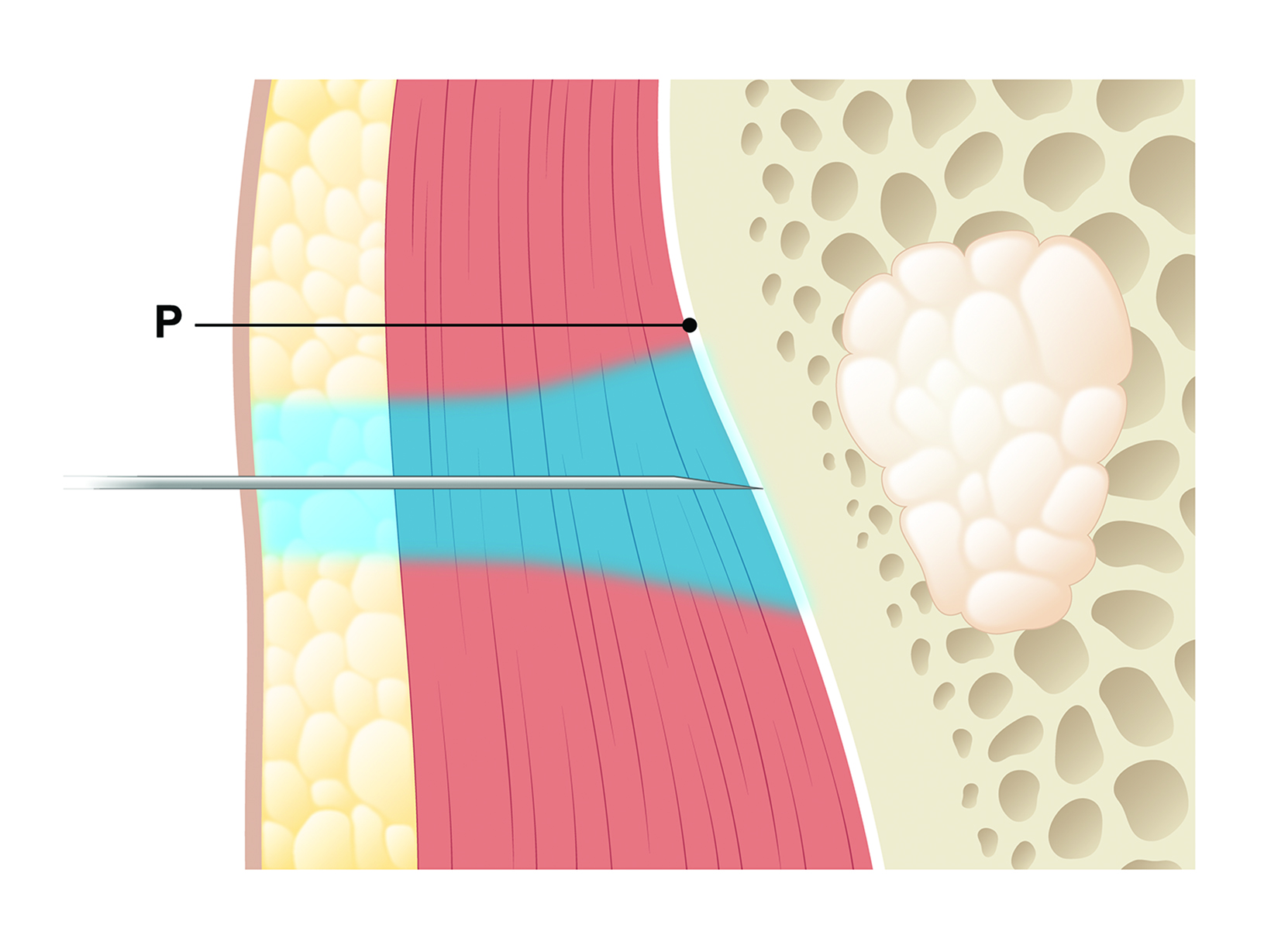
The periosteum is another well-innervated structure that can result in sharp, well-localized pain if not properly anesthetized (Figure 3). Infusing lidocaine into the subcutaneous tissues is not sufficient, and the patient will still experience pain when the needle pierces the periosteum. Special care should be taken when performing biopsies or any other bony intervention to infuse lidocaine within and over the periosteum.
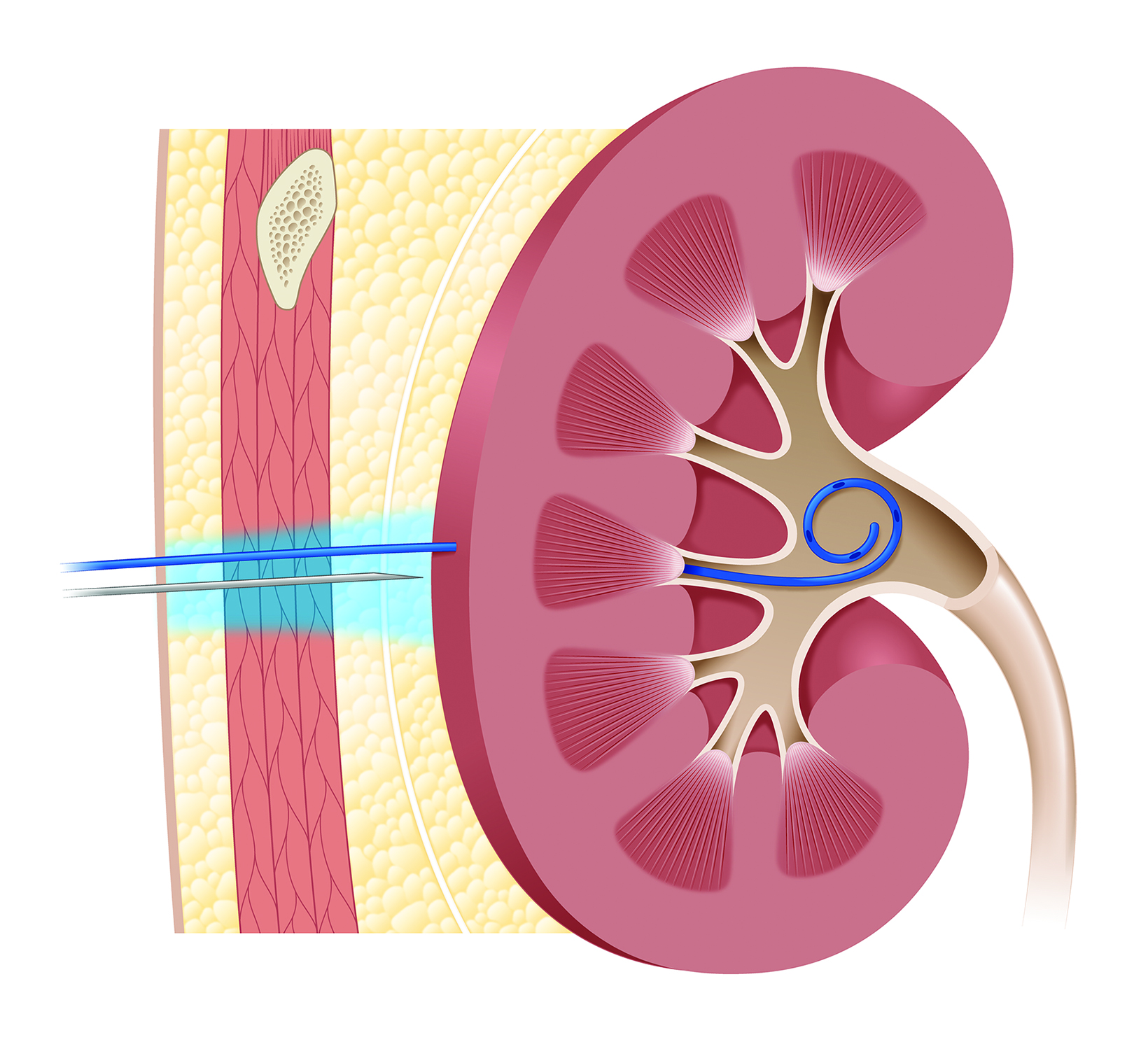
The liver, spleen, and kidneys are frequently involved in vascular and extravascular interventions. A 2019 randomized controlled study showed that subcostal approaches to hepatic interventions are less painful than intercostal approaches.40 When placing or exchanging a catheter involving the liver or kidneys, the entirety of the tract should be anesthetized, not just the subcutaneous tissues (Figure 4).
Conclusion
Inadequate pain control is an important source of patient dissatisfaction. However, using proper technique to administer local anesthesia can measurably reduce pain. Each patient represents an opportunity to improve and refine these techniques. With time and close attention to each patient, physicians may approach the goal of a nearly pain-free procedure.
References
- Culp WC Jr, Culp WC. Practical application of local anesthetics. J Vasc Interv Radiol. 2011;22(2):111-118. doi: 10.1016/j.jvir.2010.10.005
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132(3):675-684. doi: 10.1097/ PRS.0b013e31829ad1e2
- Farhangkhoee H, Lalonde J, Lalonde DH. Teaching medical students and residents how to inject local anesthesia almost painlessly. Can J Plast Surg. 2012;20(3):169-172. doi: 10.1177/229255031202000315
- Koch SC, Acton D, Goulding M. Spinal Circuits for Touch, Pain, and Itch. Annu Rev Physiol. 2018;80:189-217. doi: 10.1146/annurev-physiol-022516-034303
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760-3772. doi: 10.1172/JCI42843
- Cherobin ACFP, Tavares GT. Safety of local anesthetics. An Bras Dermatol. 2020;95(1):82-90. doi: 10.1016/j.abd.2019.09.025
- Romagnoli S, Fanelli F, Barbani F, et al. CIRSE Standards of Practice on Analgesia and Sedation for Interventional Radiology in Adults. Cardiovasc Intervent Radiol. 2020;43(9):1251-1260. doi: 10.1007/s00270-020-02536-z
- Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118(2):429-432. doi: 10.1097/01.prs.0000227632.43606.12.
- Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20(2):71-73. doi: 10.1177/229255031202000207
- Steele EA, Ng JD, Poissant TM, Campbell NM. Comparison of injection pain of articaine and lidocaine in eyelid surgery. Ophthalmic Plast Reconstr Surg. 2009;25(1):13-15. doi: 10.1097/IOP.0b013e3181912016
- Cepeda MS, Tzortzopoulou A, Thackrey M, Hudcova J, Arora Ghandi P, Schumann R. Adjusting the pH of lidocaine for reducing pain on injection. Cochrane Database Syst Rev. 2010;(12):CD006581. doi: 10.1002/14651858.CD006581.pub2
- Burns CA, Ferris G, Feng C, Cooper JZ, Brown MD. Decreasing the pain of local anesthesia: A prospective, double-blind comparison of buffered, premixed 1% lidocaine with epinephrine versus 1% lidocaine freshly mixed with epinephrine. J Am Acad Dermatol. 2006;54:128–131. doi: 10.1016/j.jaad.2005.06.043
- Malamed SF, Tavana S, Falkel M. Faster onset and more comfortable injection with alkalinized 2% lidocaine with epinephrine 1:100,000. Compend Contin Educ Dent. 2013;34(1):10-20
- Powell MF. Stability of lidocaine in aqueous solution: effect of temperature, pH, buffer, and metal ions on amide hydrolysis. Pharm Res. 1987;4:42–45. doi: 10.1023/a:1016477810629
- Johansen RB, Schafer NC, Brown PI. Effect of extreme temperatures on drugs for prehospital ACLS. Am J Emerg Med. 1993;11:450–452. doi: 10.1016/0735-6757(93)90080-u
- Hogan ME, vanderVaart S, Perampaladas K, Machado M, Einarson TR, Taddio A. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86–98.e1. doi: 10.1016/j.annemergmed.2010.12.001
- Lundbom JS, Tangen LF, Wågø KJ, et al. The influence of Lidocaine temperature on pain during subcutaneous injection. J Plast Surg Hand Surg. 2017;51(2):118-121. doi: 10.1080/2000656X.2016.1194281
- Mader TJ, Playe SJ, Garb JL. Reducing the pain of local anesthetic infiltration: Warming and buffering have a synergistic effect. Ann Emerg Med. 1994;23:550– 554. doi: 10.1016/s0196-0644(94)70076-1
- Yang CH, Hsu HC, Shen SC, Juan WH, Hong HS, Chen CH. Warm and neutral tumescent anesthetic solutions are essential factors for a less painful injection. Dermatol Surg. 2006;32:1119–1122. doi: 10.1111/j.1524-4725.2006.32254.x
- Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37– 43. doi: 10.1080/08990220600700925
- Gill HS, Prausnitz MR. Does needle size matter? J Diabetes Sci Technol. 2007;1:725-729. doi: 10.1177/193229680700100517
- Watts AC, McEachan J. The use of a fine-gauge needle to reduce pain in open carpal tunnel decompression: a randomized controlled trial. J Hand Surg [Br]. 2005;30:615–617. doi: 10.1016/j.jhsb.2005.06.021
- Wågø KJ, Skarsvåg TI, Lundbom JS et al. The importance of needle gauge for pain during injection of lidocaine. J Plast Surg Hand Surg. 2016;50:115–118. doi: 10.3109/2000656X.2015.1111223
- Ağaç E, Güneş UY. Effect on pain of changing the needle prior to administering medicine intramuscularly: A randomized controlled trial. J Adv Nurs. 2011;67:563–568. doi: 10.1111/j.1365-2648.2010.05513.x
- Gajraj NM, Pennant JH, Watcha MF. Eutectic mixture of local anesthetics (EMLA) cream. Anesth Analg. 1994;78:574–583
- Cárceles MD, Alonso JM, García-Muñoz M, Nájera MD, Castaño I, Vila N. Amethocaine-lidocaine cream, a new topical formulation for preventing veno-puncture-induced pain in children. Reg Anesth Pain Med. 2002;27:289–295.
- Kuwahara RT, Skinner RB. Emla versus ice as a topical anesthetic. Dermatol Surg. 2001;27:495–496. doi: 10.1046/j.1524-4725.2001.00343.x
- van Wijk AJ, Hoogstraten J. Anxiety and pain during dental injections. J Dent. 2009;37:700–704. doi: 10.1016/j.jdent.2009.05.023
- Okawa K, Ichinohe T, Kaneko Y. Anxiety may enhance pain during dental treatment. Bull Tokyo Dent Coll. 2005;46:51–58. doi: 10.2209/tdcpublication.46.51
- Uman LS, Chambers CT, McGrath PJ, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: An abbreviated Cochrane review. J Pediatr Psychol. 2008;33:842–854. doi: 10.1093/jpepsy/jsn031
- Höfle M, Hauck M, Engel AK, Senkowski D. Viewing a needle pricking a hand that you perceive as yours enhances unpleasantness of pain. Pain. 2012;153:1074–1081. doi: 10.1016/j.pain.2012.02.010
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155(2):210-216. doi: 10.1016/j.pain.2013.12.010
- Nanitsos E, Vartuli R, Forte A, Dennison PJ, Peck CC. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54:94-100. doi: 10.1111/j.1834-7819.2009.01100.x
- Baxter AL, Cohen LL, McElvery HL, Lawson ML, von Baeyer CL. An integration of vibration and cold relieves venipuncture pain in a pediatric emergency department. Pediatr Emerg Care. 2011;27(12):1151-1156. doi: 10.1097/PEC.0b013e318237ace4
- Patel BK, Wendlandt BN, Wolfe KS, et al. Comparison of Two Lidocaine Administration Techniques on Perceived Pain from Bedside Procedures — A Randomized Clinical Trial. Chest. 2018;154(4):773-780. doi: 10.1016/j.chest.2018.04.018
- Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: Lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231–1233. doi: 10.1016/j.jaad.2011.04.011
- Michael AA, Moorjani GR, Peisajovich A, Park KS, Sibbitt WL Jr, Bankhurst AD. Syringe size: Does it matter in physician-performed procedures? J Clin Rheu- matol. 2009;15:56–60. doi: 10.1097/RHU.0b013e31819c1fc4
- Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31(1):36-40. doi: 10.1016/s0196- 0644(98)70278-1
- Serour F, Mandelberg A, Mori J. Slow injection of local anaesthetic will decrease pain during dorsal penile nerve block. Acta Anaesthesiol Scand. 1998;42(8):926-928. doi: 10.1111/j.1399-6576.1998.tb05351.x
- Pezeshki Rad M, Abbasi B, Morovatdar N, Sadeghi M, Hashemi K. Pain in percutaneous liver core-needle biopsy: a randomized trial comparing the intercos- tal and subcostal approaches. Abdom Radiol (NY). 2019;44(1):286-291. doi: 10.1007/s00261-018-1704-z
Citation
M H, Y L, DL K.Minimizing the Pain of Local Anesthetic Injection. Appl Radiol. 2024; (1):16-21.
January 24, 2024