Koios Medical Smart Ultrasound AI Software Gets FDA Nod
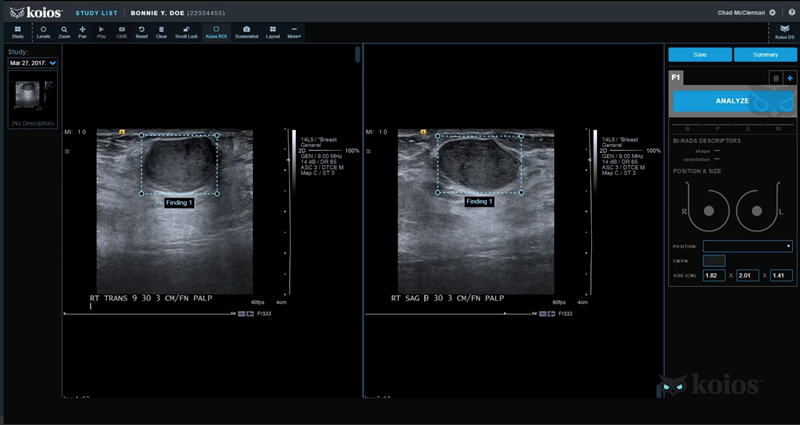 Koios DS, an artificial intelligence (AI) based software platform used to diagnose thyroid and breast cancer from Koios Medical, has received US FDA clearance. The new system, built using ultrasound data from a network of 48 sites around the world, aids physicians in accurately diagnosing disease and improves speed to treatment while reducing avoidable surgical procedures.
Koios DS, an artificial intelligence (AI) based software platform used to diagnose thyroid and breast cancer from Koios Medical, has received US FDA clearance. The new system, built using ultrasound data from a network of 48 sites around the world, aids physicians in accurately diagnosing disease and improves speed to treatment while reducing avoidable surgical procedures.
“Thyroid DS is a game changer for thyroid ultrasound imaging. Not only will it aid diagnosis of thyroid cancer it will allow for more standardized and reproductive reporting of thyroid nodules preventing potentially unwarranted biopsies on benign lesions,” says Dr. Shah Islam, National Hospital for Neurology & Neurosurgery, London.
In the US alone, breast and thyroid cancers combine for over 375,000 diagnosed cases annually. Over 2.2 million biopsy procedures of breast and thyroid tissue are performed, yet tens of thousands of cancers still go undetected. Breast cancer is the second most diagnosed cancer in women worldwide. An ultrasound exam is the standard of care for women with dense breast tissue.
Thyroid disease is one of the most complex and challenging radiological interpretations. Diagnostic uncertainty drives a high level of variability across physicians and results in surgical procedures, downstream cost, risk of complication and physician burn-out. The company performed studies that proved physicians using Koios DS software dramatically improved accuracy, consistency, and efficiency.
Physicians’ thyroid cancer detection rates jumped by up to 14% while simultaneously reducing false positive biopsy orders by over 35%. Interpretation variability was lowered by over 50% and time spent per case dropped by 24%.
“The typical decision-making paradigm relies on tradeoffs; trading sensitivity for specificity, efficiency for thoroughness, but the only thing enforcing this paradigm is the inability to shift off of these tradeoff curves in place of shifting along them. This novel software demonstrates that using AI for decision support physicians can make clinically meaningful shifts in performance improving interpretation efficacy and diagnostic performance, improving sensitivity and reducing false positives. It is exciting to bring these innovations to physicians and ultimately their patients to elevate the level of care broadly,” says Dr. Lev Barinov, VP of Clinical Excellence.
The patented AI software aligns Koios DS AI generated findings directly to the American College of Radiology’s BI-RADS and TI-RADS rating systems as well as the American Thyroid Association’s system for tissue classification, scoring and patient management.
The AMA recently announced new CPT Category 3 codes for whenever Koios DS software is utilized interpreting, classifying and reporting traditional ultrasound exams.
“The ability of physicians and health systems to now code and bill for the use of this innovative and effective technology will most certainly accelerate adoption, putting the software into the hands of physicians for the benefit of patients nationwide,” says Graham Anderson, Koios Medical CFO.
The rigor of the FDA clearance process required that Koios DS be proven to accurately interpret images from all major ultrasound hardware manufacturers. The software is compatible with major PACS workstation viewers and integrated into GE Healthcare’s LOGIQ E10 ultrasound scanner. The system enables real-time decision-making with results that can be exported directly into a patient's record and all major reporting systems, reducing errors and saving time.