Generalized Lymphatic Anomaly
Case Summary
An adolescent was referred to pediatric pulmonology for difficulty recovering from pneumonia the preceding year, with multiple prior episodes of pneumonia. The patient presented with cough, dyspnea, chest pain, abdominal pain, and extremity bruising. Pulmonary physical examination findings included asymmetric breath sounds predominantly in the left lung; crackles in the left base; and suprasternal, intercostal, and subcostal retractions. Imaging revealed mediastinal and interstitial thoracic abnormalities in addition to pericardial and pleural effusions. The patient underwent a pericardiocentesis with drain placement, pleural effusion drainage, and biopsy of the mediastinal soft tissue. The biopsy demonstrated irregular, dilated lymphatic channels with positivity for CD31 and D2-40, which are diagnostic of a lymphatic malformation. Further imaging revealed additional areas of involvement.
Imaging Findings
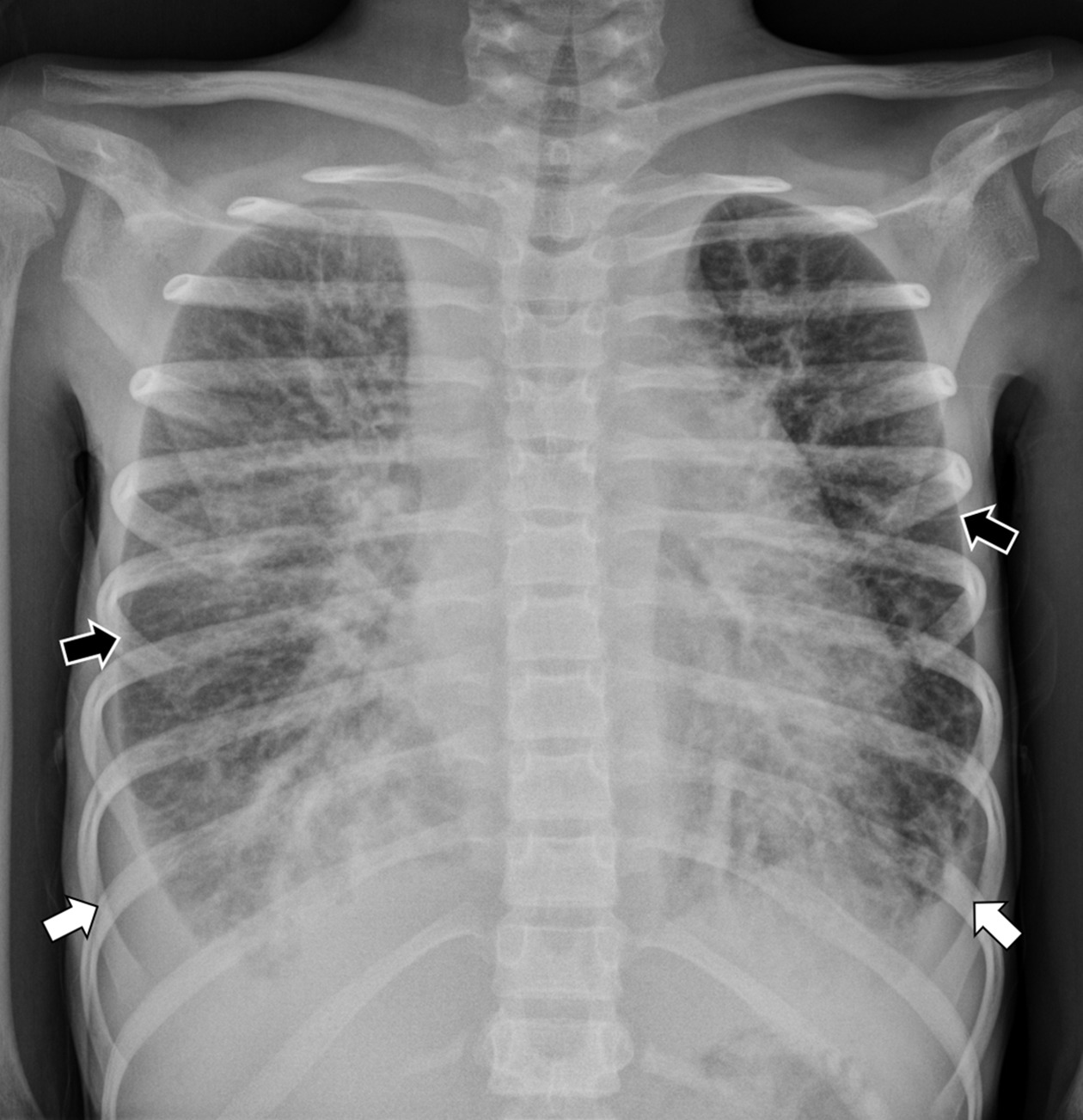
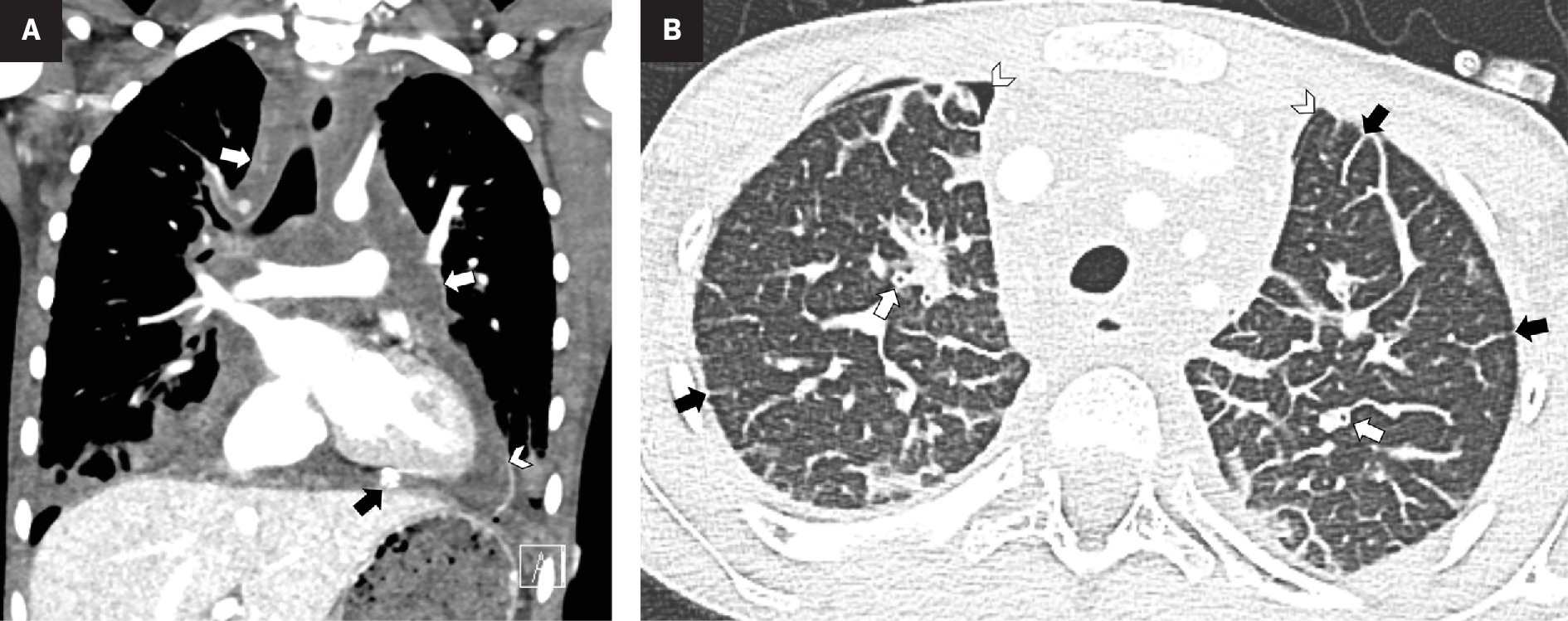
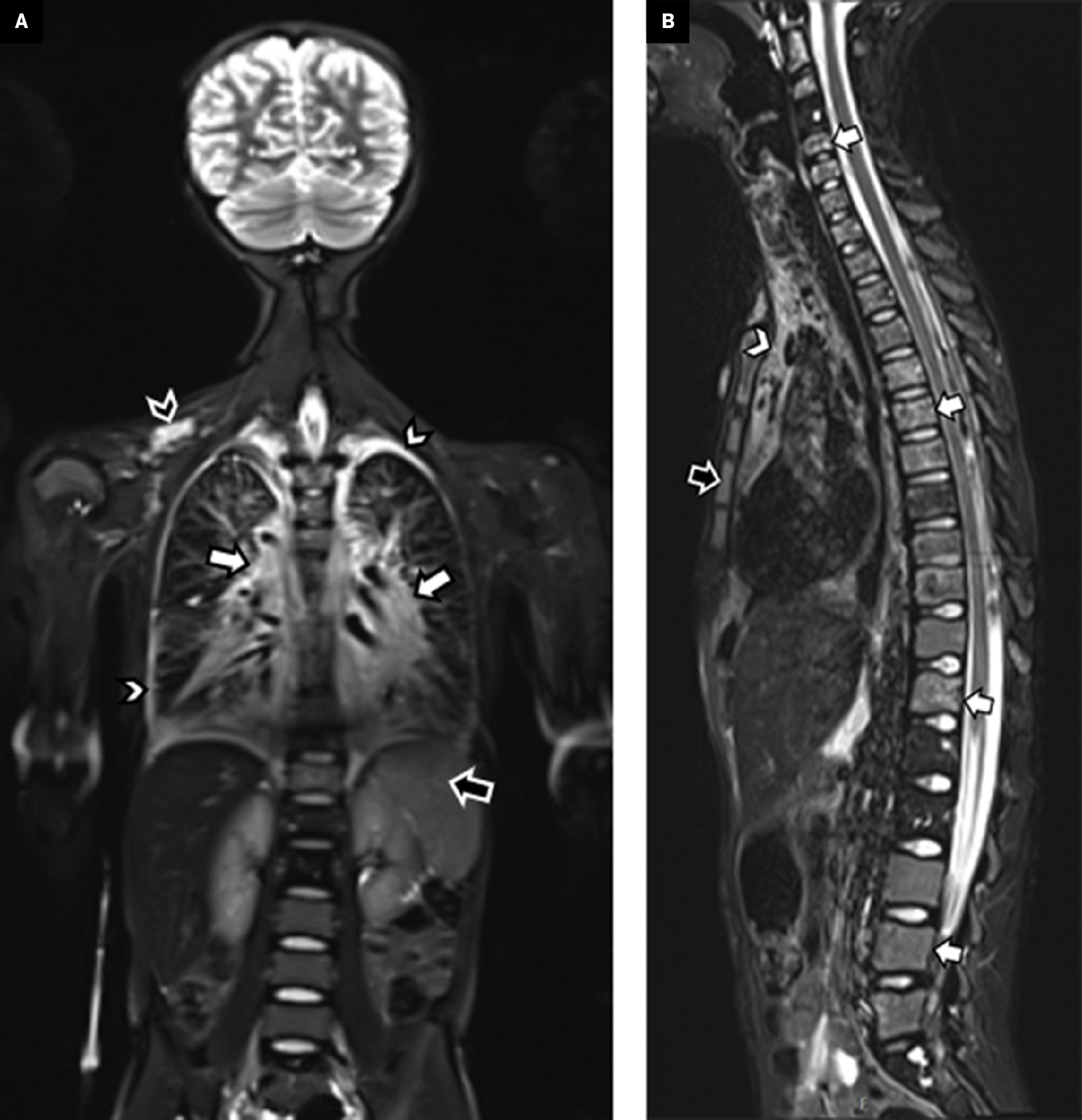
Chest radiograph showed an enlarged cardiothymic silhouette with interstitial opacities and small bilateral pleural effusions ( Figure 1 ). An echocardiogram showed a large pericardial effusion. Following pericardiocentesis and bilateral chest tube placement, chest CT ( Figure 2 ) showed infiltrative low-density mediastinal soft tissue extending along the bronchovascular bundles into the lungs where there was diffuse thickening of the interlobular septa. Whole-body MRI ( Figure 3 ) also showed diffuse thoracic vertebral T2 hyperintense lesions, abnormal T2 hyperintensity throughout the mediastinum and lungs, as well as small pleural effusions. The appendicular and axial skeleton were involved with the majority of the spine and long bones having patchy confluent marrow signal abnormality with metaphyseal predilection. There were scattered lobulated T2 hyperintense soft-tissue lesions. The spleen had serpiginous T2 hyperintense signal as well. The findings overall were compatible with multifocal, multicompartmental lymphatic malformations.
Frontal radiograph of the chest shows an enlarged cardiothymic silhouette. Within the lungs are small pleural effusions (white arrows) and diffuse interstitial thickening (black arrows).
Contrast-enhanced CT of the chest. Coronal soft-tissue window ( A ) shows infiltrative hypodense mediastinal soft tissue (white arrows) and small pleural effusions. There is a pericardial effusion (arrowhead) with a pericardial drain partially visible (black arrow). Axial lung window ( B ) shows interlobular septal thickening (black arrows) and peribronchial cuffing (white arrows). Small bilateral pneumothoraces (arrowheads) are iatrogenic related to the mediastinal biopsy and pericardiocentesis.
Whole-body MRI. Coronal short tau inversion recovery (STIR) imaging through the torso ( A ) shows a diffusely increased signal within the mediastinum and tracking along the bronchovascular bundles into the hila (white arrows). Small pleural effusions are also evident (white arrowheads). Within the spleen are multiple serpiginous hyperintense foci (black arrow) related to splenic involvement. A lobulated hyperintense soft-tissue lesion is within the right supraclavicular region (black arrowhead), compatible with a soft-tissue lymphatic malformation. A sagittal STIR ( B ) image of the spine shows multilevel hyperintense lesions, predominately within the vertebral bodies (white arrows) and sternum (black arrow). The hyperintense mediastinal signal is also evident (arrowhead).
Diagnosis
Generalized lymphatic anomaly (GLA).
The primary differential diagnoses for multifocal lymphatic malformations and bone lesions are Gorham-Stout disease and Kaposiform lymphangiomatosis (KLA). KLA is more aggressive than GLA and is even more rare. The two must be differentiated from each other histologically.1 GLA and Gorham-Stout disease share many features. However, Gorham-Stout will have associated osteolysis, hence its other name vanishing bone disease .2 The differential diagnosis of pulmonary lymphangiomatosis also includes congenital pulmonary lymphangiectasia and primary and secondary lymphedema. If the bone lesions were the presenting abnormality, differential considerations would include Langerhans cell histiocytosis, metastatic disease, and polyostotic fibrous dysplasia.
Discussion
GLA is a rare congenital condition characterized by multiple lymphatic malformations (abnormal proliferation, dilation, and anastomosis of lymphatic channels) throughout the body. It may involve the bones, thoracic and abdominal viscera, and soft tissues.3 The former term diffuse systemic lymphangiomatosis was renamed GLA. The disease often presents in childhood, but may also be diagnosed in adults and seems to have no preference for sex.4 Due to the rarity of GLA and its indolent course, delayed diagnosis is common.
However, patients with pulmonary involvement can experience symptoms long before their diagnosis. Common symptoms include cough, wheezing, milky sputum, and shortness of breath, potentially leading to misdiagnosis with asthma.5 Pleural effusions, pericardial effusions, and respiratory infections often occur. The pleural effusions are attributed to the compression of lymphatic channels by mediastinal masses. Chylothorax, chyle accumulation in the pleura, is common among these patients. Chyloptsis, hemoptysis, chylopericardium, protein-wasting enteropathy, peripheral lymphedema, and disseminated interstitial coagulopathy may also occur. Bone involvement of the axial or appendicular skeleton occurs in up to 75% of patients with GLA.6
Chest radiographs typically show pulmonary bilateral interstitial thickening associated with pericardial and/or pleural effusions.6 Mediastinal widening or soft-tissue masses may also be observed. Whole-body MRI can determine the extent of organ involvement.3 Potential thoracic findings include interlobular septal thickening tracking along the bronchovascular bundles, ground-glass opacities, perihilar infiltration, pleural effusions, and infiltration and thickening of the mediastinal soft tissues. MRI may also show pleural effusions and multifocal lymphatic malformations of the spleen, bones, and soft tissues.7
Radiographs of GLA may identify lucent bone lesions. Osteolytic lesions due to a general lymphatic anomaly are best appreciated via CT, but MRI is often necessary for accurate assessment of bone marrow changes and soft-tissue involvement.8 Lymphangiography reveals lesions of the thoracic duct, dilated lymphatic channels, and lymphangiomas within the bones and lungs. It also helps differentiate malformations from malignant tumors and evaluate the extent of the disease.6 However, the technique used can cause pulmonary complications such as pulmonary oil embolism from blockage of the thoracic duct and inflammatory responses to ethiodized oil.
A biopsy of lymphatic areas or soft-tissue masses aids in definitive diagnosis. Histopathology of the lymphatic channels demonstrates anastomosing endothelial cells along pulmonary lymphatic routes.6 These endothelial cells within lymphatic channels will stain positive for CD31 and D2-40 in patients with GLA. CD31 is a vascular marker for blood vessel endothelium and positivity indicates angiogenesis. D2-40 is a marker specific to lymphatic endothelium; a positive stain signifies lymphatic proliferation and differentiation.9
GLA has no established cure. Current treatment regimens focus on supportive care and symptomatic relief from the compressive effects of mediastinal masses and chylous fluid accumulations. Surgical resection of lymphatic malformations is rare as it is difficult to separate lymphatic channels from surrounding structures.6 Treatment options include low-fat, medium-chain triglyceride diets, corticosteroids, interferon-α, sirolimus, propranolol, somatostatin, and bevacizumab.10 Propranolol, sirolimus, and bevacizumab work to repress lymphangiogenesis by binding vascular endothelial growth factor or its receptor. Sirolimus, in particular, has been beneficial in reducing lymphatic proliferation.4
Conclusion
GLA is a rare condition of multisystemic lymphatic proliferation and dilation often involving the bones, viscera, and mediastinum. Due to the generalized initial symptoms of this disease and its slow course, diagnosis is often delayed. Milky sputum, recurrent respiratory infections, chylothorax, and bony involvement are strong indicators of GLA. The primary goal of imaging is to determine the extent of lymphatic malformations throughout the body and which organ symptoms are involved. While there is no definitive cure, sirolimus treatment has shown promise in symptom management.
References
Citation
O’Connor Z, McBee MP. Generalized Lymphatic Anomaly. Appl Radiol. 2024;(5):29 - 32.
doi:10.37549/AR-D-24-0034
October 1, 2024