Focused Ultrasound for Ablation in Neurosurgery — Present Use and Future Directions
Images
Focused ultrasound (FUS) as a therapeutic modality for the treatment of neurological conditions has seen a rapid expansion over the past decade due to its ability to produce controlled and precise effects non-invasively. FUS has multiple mechanisms of action, but at higher frequencies, thermal ablation is predominant and is capable of precise and controlled lesioning of brain tissue. In particular, transcranial magnetic resonance-guided focused ultrasound surgery (MRgFUS), a non-invasive ablative modality has become a well-established tool in functional neurosurgery for movement disorders such as essential tremor (ET) and Parkinson Disease (PD). Since its FDA approval in 2016, MRgFUS has gained popularity amongst researchers, clinicians, and patients. Ongoing studies to evaluate additional indications are underway. Ongoing clinical trials include treatment of psychiatric illness, chronic pain, and epilepsy. Radiologists should be familiar with the expected neuroimaging findings following treatment and the entities for which this is utilized and being explored.
A Brief History of Focused Ultrasound
Although ultrasound was discovered in the late 1800s, the invention of FUS is attributed to Johannes Gruetzmacher, who placed curved quartz on a piezoelectric generator to concentrate waves in 1935. Initial trials in humans targeted deep structures for movement disorders, but lesions were imprecise prior to the advent of modern imaging. Furthermore, large portions of skull were removed to mitigate wave distortion and surface heating. In 1998, the use of MRI and a helmet equipped with two arrays and 64 elements was shown to transmit pulsed sonication through a piece of a human skull to induce tissue destruction in an animal model, which catapulted FUS into widespread use.
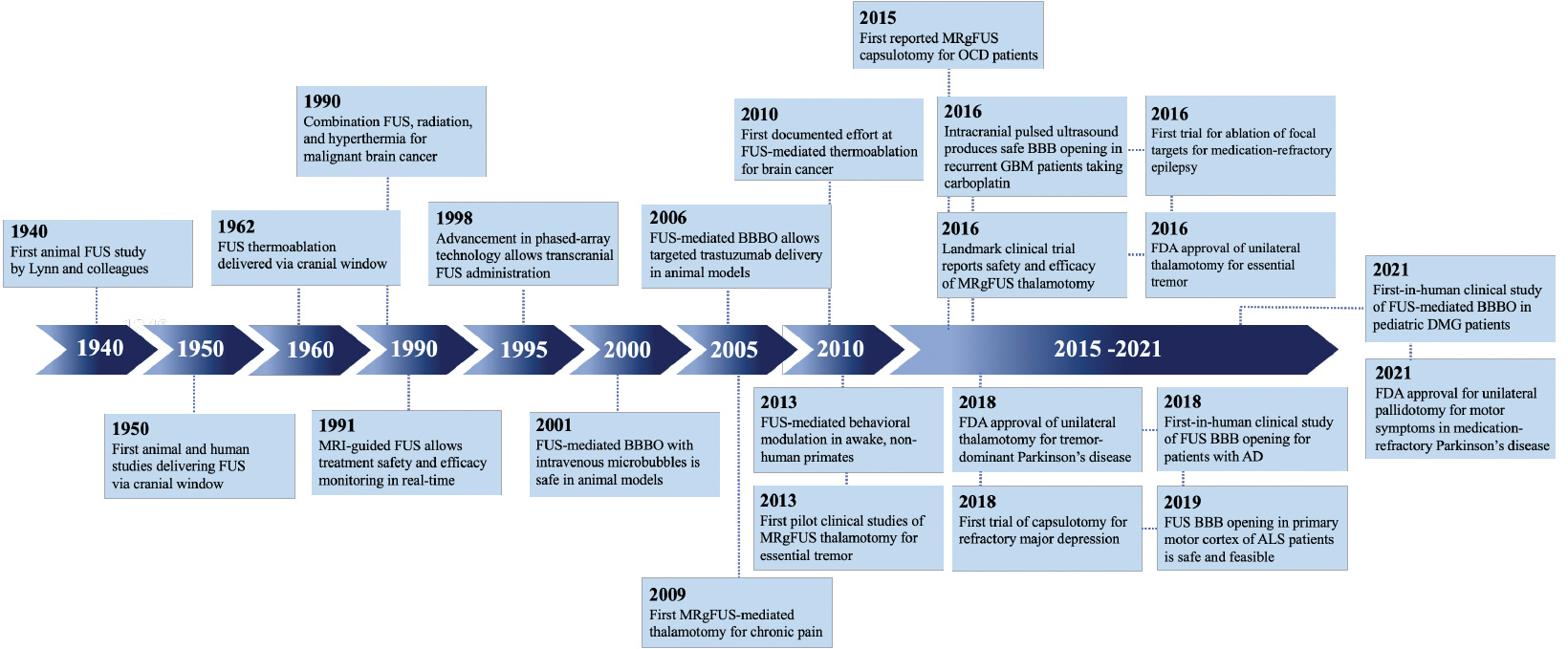
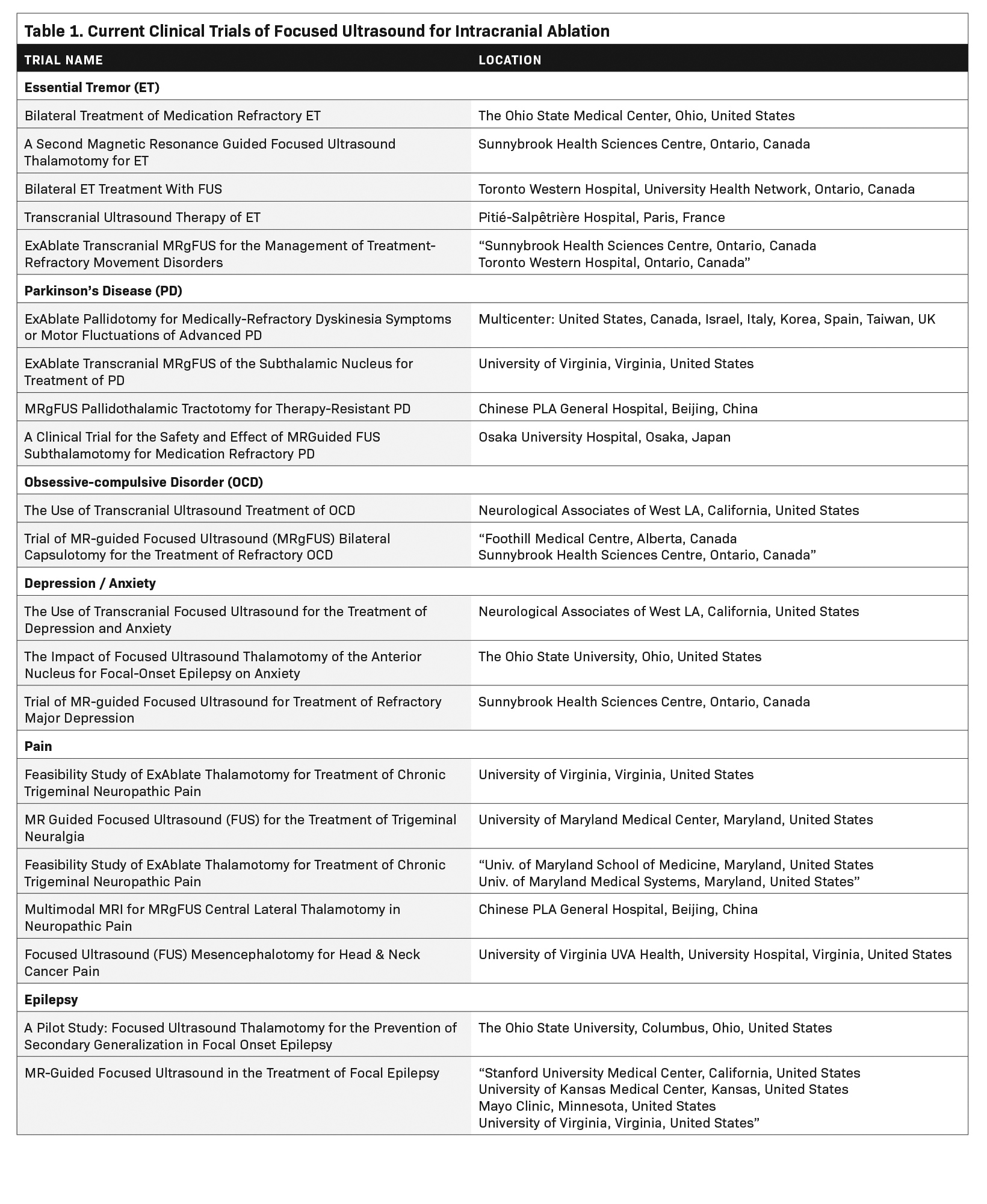
Since then, myriad developments such as real-time MRI guidance, have improved the safety and efficacy of FUS ablation (Figure 1). As of this writing, three neurological indications have been FDA-approved for MRI-guided FUS (MRgFUS): thalamotomy for ET, thalamotomy for tremor-dominant PD, and pallidotomy for the motor symptoms of PD. Multiple additional indications are currently being investigated (Table 1).
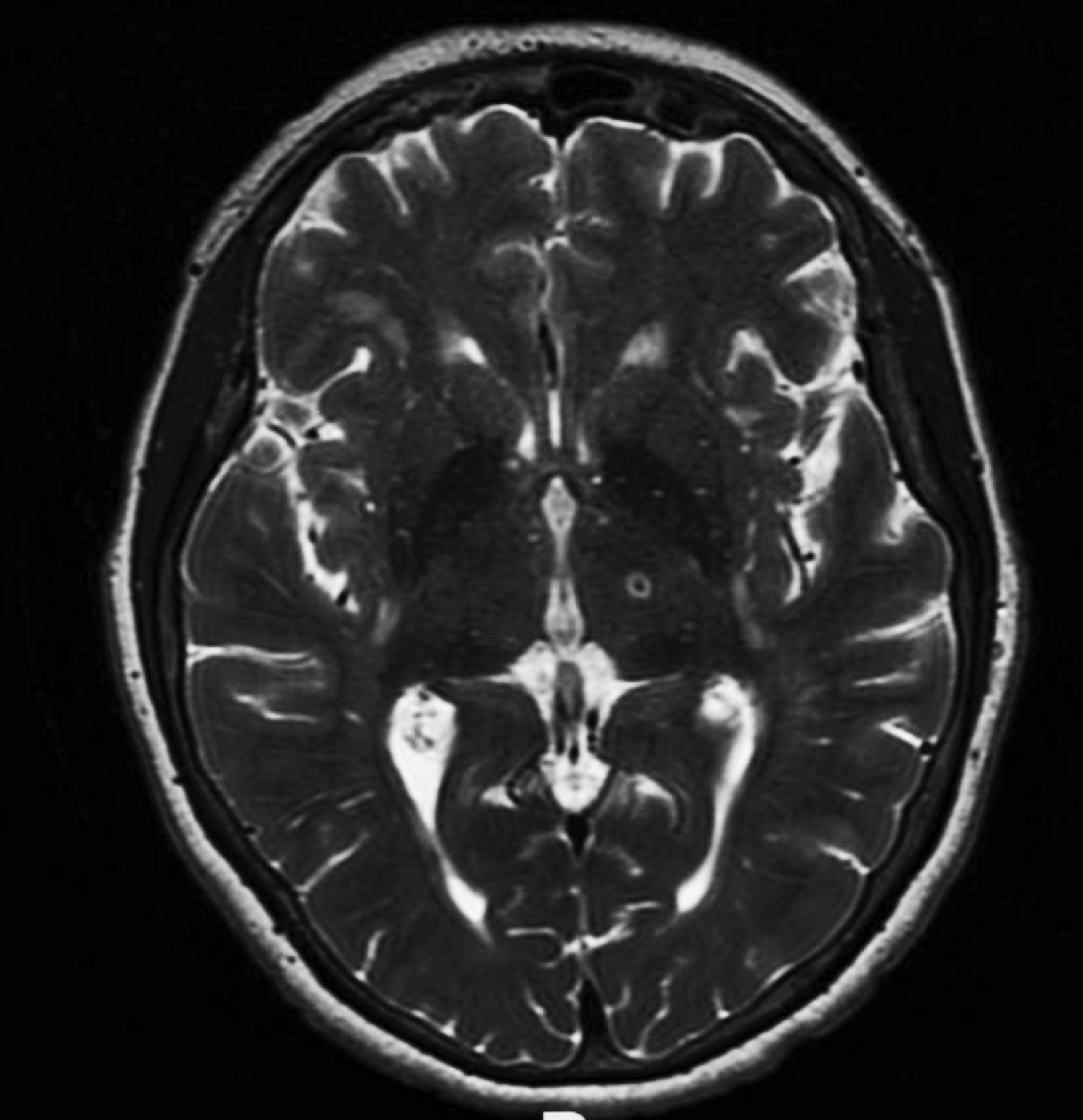
Radiographic findings shortly after FUS ablation have been well characterized as three concentric zones representing target coagulation necrosis (Zone I), cytotoxic edema (Zone II), and perilesional vasogenic edema (Zone III).3 On T2 images, Zone I is seen as a central hypointense region. Zone II is strongly hyperintense region with a hypointense rim, while Zone III is a diffuse hyperintense region beyond Zone II (Figure 2). These regions appear 24 hours to 1 week after treatment and disappear over 1-3 months, leaving a single, minimally T2 hypointense lesion. This T2 pattern was also observed by researchers evaluating MRgFUS-induced lesions on T2, T1, diffusion weighted imaging, and susceptibility weighted imaging (SWI) at 3, 30, and 180-day timepoints after treatment.4 Over a longer time horizon, once T2 signal resolves, a shrinking or disappearance of the lesion can continue to be visualized through day 180 on T1 scans. MRgFUS-induced lesions have the most longevity on SWI, which is sensitive to even subtle hemosiderin remnants caused by the initial treatment.
Mechanism of Action for Ablation
The ablative action of focused ultrasound is dependent on frequency, which leads to either thermal or mechanical tissue destruction. At higher frequencies (~650 kHz), thermal ablation is predominant. The United States Food and Drug Administration (FDA)-approved hemispherical transducer (ExAblate 4000; Insightec, Inc) can achieve peak temperatures of 51°C to 60°C under continuous visual MR-guidance and MR-thermometry with an accuracy of <2mm.
High incident angles (>20°) prohibit targets closer to the skull from successful treatment; thermal ablation can only be applied to more central brain regions. Thick and poorly homogeneous skulls limit the penetration of ultrasound. Preoperative CT is obtained to assess patient-specific metrics such as skull thickness and skull homogeneity as quantified by skull density ratio (SDR).
An SDR below 0.4 is considered inconducive to optimal thermal lesioning and FDA-labeling includes only patients with an SDR of 0.4 or higher.
At a single-center study, 50% of patients evaluated had a skull score under 0.4, suggesting that a significant number of patients may be ineligible for MRgFUS owing to skull characteristics.
Lower frequencies (around 220 kHz) produce therapeutic mechanical energy by interacting to rapidly expand and contract entrapped gas in a process called cavitation. Cavitation to the point of tissue destruction is focal and leaves the surrounding tissue intact. Lower frequencies are less susceptible to acoustic absorption and higher incident angles, expanding the potential reach of MRgFUS to the entire intracranial space. This remains an area of active research.
Current FDA-approved Indications
MRgFUS ablation has become a well-established tool in functional neurosurgery for movement disorders such as ET and PD. Given the small size of tissue targets and their central location within the skull, as well as an aged patient population with higher operative risk, movement disorders approximate ideal indications for noninvasive thermal ablation.
Essential Tremor
In July 2016, thalamotomy for refractory ET became the first FDA-approved intracranial use of MRgFUS. Essential tremor is the most common cause of action tremor in adults and remains progressive with no disease-modifying agents. Current first-line treatment for the condition consists of medical therapy; however, approximately 30% of patients see no therapeutic benefit.
Second-line treatment includes combination drug therapy. Patients failing adequate trials of medical therapy may be offered surgical options, which include treating the ventral intermediate nucleus of the thalamus with deep brain stimulation (DBS) or thalamotomy.
Treatment is largely unilateral owing to concerns for increased complications with bilateral thalamotomy, but recent evidence suggests a bilateral staged plan can be safe and effective.
Essential tremor remains the subject of numerous active clinical trials to expand and optimize its treatment.
Parkinson Disease
Parkinson disease is the second most common neurodegenerative disease, with a steadily increasing global prevalence. More than 6 million individuals are affected, a 2.5-fold increase over the past generation.
This number is projected to double again by 2040, even to as high as 17 million, given increasing longevity, declining smoking rates, and increasing industrialization.
In 1960, 50 patients with PD were among the first treated with FUS, a 14-hour procedure that required craniotomy, with only temporary improvement at best.
As the drug L-dopa was developed, medical management became primary. In 2018, thalamotomy for tremor-dominant PD received FDA approval, becoming the only additional intracranial indication for FUS.
For patients with treatment-resistant PD, DBS has largely replaced conventional lesioning. The internal globus pallidus is commonly targeted in DBS, but its lateral location can be challenging for thermoablation. Nevertheless, MRgFUS pallidotomy has been shown to decrease tremor and improve motor function; FDA approval for pallidotomy has been granted.
A promising area of study is lesioning of the pallidothalamic tract for chronic therapy-resistant PD. A recent small study showed significant reductions in tremor and rigidity.
Currently, multiple clinical trials are underway worldwide to study thermoablation targets for PD.
Frontier Indications
Psychiatric Diseases
MRgFUS capsulotomy is being studied as a potential treatment for obsessive–compulsive disorder (OCD), depression, and anxiety. Obsessive compulsive disorder is related to an imbalance of excitatory and inhibitory pathways in the corticostriatal– thalamocortical circuit. MRgFUS treatment has focused on the anterior limb of the internal capsule. Two trials studied MRgFUS anterior capsulotomy for medically refractory OCD with moderate reduction in symptomatology in some patients.
Major depressive disorder (MDD) is highly prevalent and treatment-refractory in one-third of patients and is often comorbid with anxiety and other psychiatric illness. It is a heterogeneous disorder, implicating numerous structural and functional brain circuits, with historical surgical targets including the internal capsule, anterior cingulate, subcaudate tracts, and limbic system.
Clinical trials are underway to further evaluate the efficacy of MRgFUS for MDD and anxiety.
Chronic Pain
Pain lasting more than three months may be as high as 10% in the US population. Focused ultrasound was approved by the FDA in 2012 for the treatment of pain from osseous metastases, and clinical trials to investigate intracranial applications of MRgFUS for pain are ongoing.
Anterior cingulate, brainstem, spinal cord, and pituitary gland targets have all been considered, but the thalamus remains a principal target given its role in the relay of ascending nociceptive input from neurons of the spinal thalamic tract to key cortical areas. Bilateral central lateral thalamic nuclei thermoablation in a small study produced pain relief in >50% of subjects at 1 year.
Treatment of the neuropathic pain associated with trigeminal neuralgia with MRgFUS bilateral medial thalamotomy is being studied.
Chronic pain is a heterogeneous disease with multifactorial effectors, and while MRgFUS will not be a cure-all it will likely join the broad armamentarium of medical, percutaneous, and surgical treatments.
Epilepsy
Two clinical trials of MRgFUS in epilepsy are ongoing: one to investigate ablation of the anterior nucleus of the thalamus to prevent secondary generalization in focal onset epilepsy; the other in patients with comorbid moderate-to-severe anxiety. MRgFUS for epilepsy remains a nascent field for study.
Conclusion
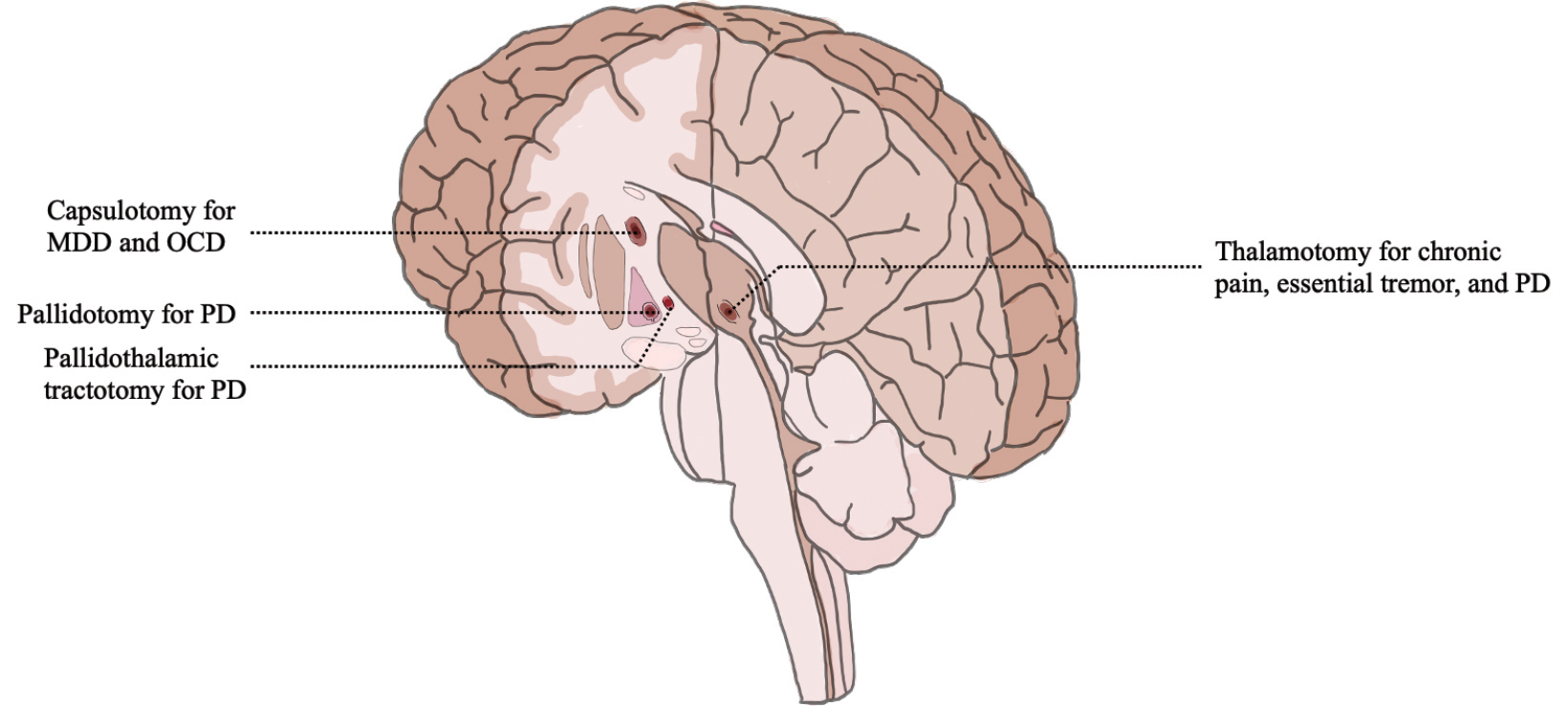
Since its FDA approval in 2016, FUS has gained popularity among researchers, clinicians, and patients. Its utility is particularly relevant in neurological diseases where small, deep lesions provide a large effect in a multitude of pathological conditions (Figure 3). MRgFUS thalamotomy for ET and thalamotomy or pallidotomy for PD are becoming increasingly common. Initial studies of safety and efficacy for additional indications ranging from depression to neuropathic pain are encouraging and may soon garner regulatory approval.
Given the aging US population as well as the growing prevalence of diseases considered for treatment, MRgFUS should solidify its role as a noninvasive ablative therapeutic option for an increasing number of patients. Diagnostic and interventional radiologists should be familiar with the appearances of lesions on neuroimaging studies and the role this therapeutic modality can play in patient care.
References
Citation
N Y, G DLS, M T, Z E, A L, C-C W, G B. Focused Ultrasound for Ablation in Neurosurgery — Present Use and Future Directions. Appl Radiol. 2023;(4):14-19.
June 23, 2023