FDA Approves GE HealthCare Flyrcado PET Tracer for Diagnosing Coronary Artery Disease
Images
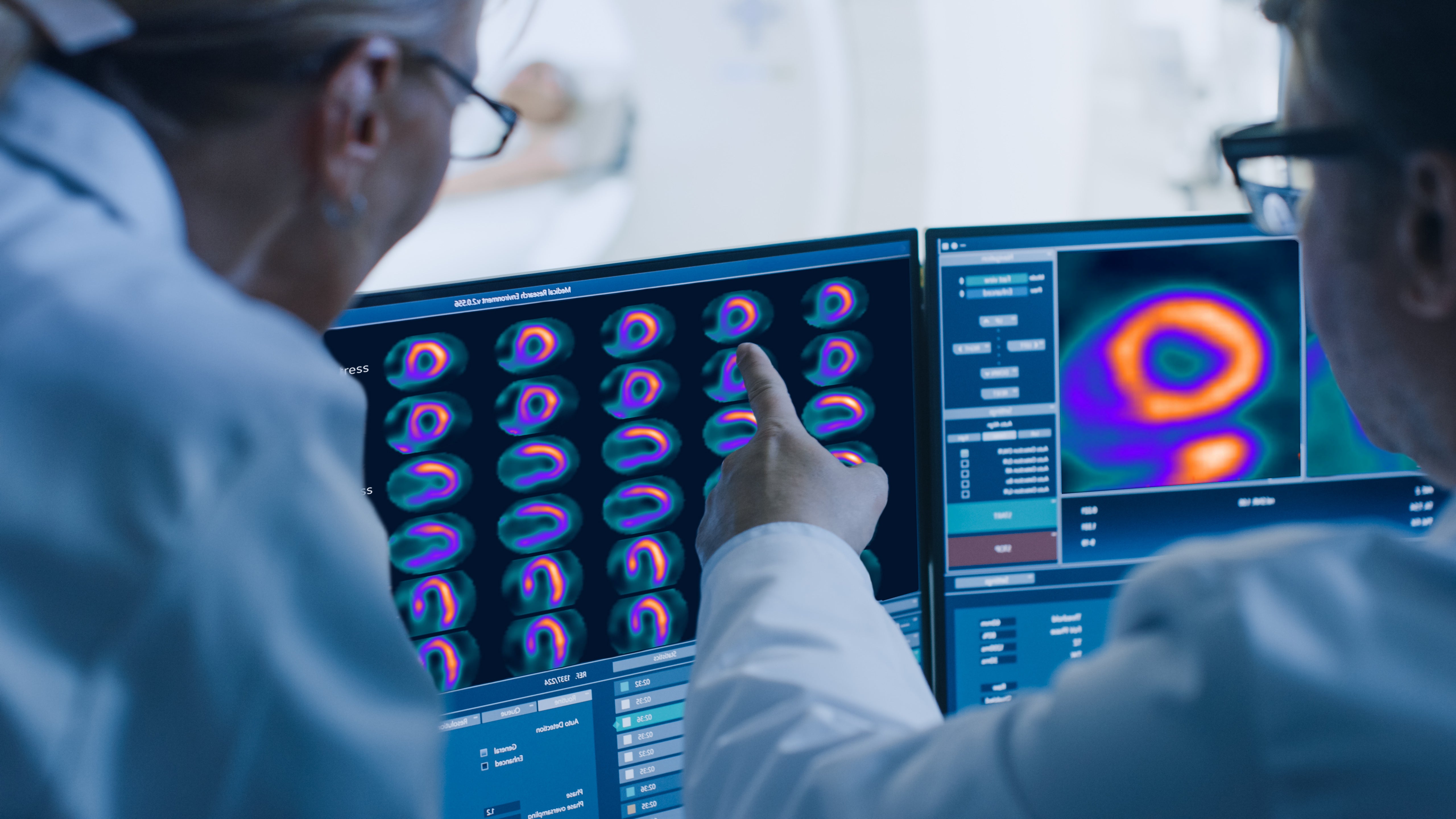
GE HealthCare announced FDA approval of Flyrcado (flurpiridaz F 18) injection, a PET MPI agent for the detection of coronary artery disease (CAD). Indicated for patients with known or suspected CAD, Flyrcado delivers higher diagnostic efficacy compared to single-photon emission computed tomography (SPECT) MPI, the predominant procedure used in nuclear cardiology today. Flyrcado, which can be manufactured in an offsite pharmacy and delivered as a ready-to-use unit dose, has the potential to expand clinician and patient access to PET MPI, including improving diagnostic accuracy in difficult-to-image patients such as those with a high body mass index (BMI) and women.
With a half-life of 109-minutes—significantly longer than existing PET MPI tracers—Flyrcado removes the need for on-site tracer production and generator maintenance and enables distribution to a wide network of hospitals and imaging centers. This longer half-life also means Flyrcado brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD, enabling the most robust protocol for evaluating ischemia in patients. Furthermore, clinicians would have the ability to rescan a patient during the same imaging session in the event of technical difficulties, rather than rescheduling an additional scan.
Dr Jamshid Maddahi, MD, FACC, MASNC, principal investigator of the Flyrcado clinical trials, clinical professor of medicine (cardiology) and molecular and medical pharmacology (nuclear medicine) at UCLA School of Medicine and director of the Biomedical Imaging Institute, said, “Flyrcado is the most exciting development in the field of nuclear cardiology over the past few decades. Although PET MPI as a modality enables high diagnostic accuracy as compared to SPECT MPI, only a minority of annual PET scans in the US are PET MPI because of limited access to the currently available PET tracers—which may be addressed with the introduction of Flyrcado. I am excited for this new radiotracer and its potential impact, as a game changer, for diagnosing the disease with the highest mortality rate in the world.”
During the multicenter international AURORA Phase III trial, flurpiridaz F 18 was compared with both invasive coronary angiography as a standard of truth to determine diagnostic efficacy in detecting CAD, as well as with SPECT MPI. Around six million MPI procedures are undertaken each year in the US to show blood flow through the heart muscle, and evaluate the presence, extent and degree of myocardial ischemia or infarction.
Dr Mouaz Al-Mallah, MD, MSc, MASNC, immediate Past President of the American Society of Nuclear Cardiology (ASNC) and Director of Cardiac PET at Houston Methodist Hospital, said “Given the desirable properties of flurpiridaz F 18, both in terms of myocardial uptake and fluorine-18 imaging characteristics, Flyrcado represents a favorable combination of imaging agent pharmacology and convenience to imaging institutions and patients. There are new frontiers for cardiac PET that this tracer can achieve; it can be ordered as a unit dose, and it offers the flexibility to perform exercise stress testing. We expect new imaging centers to be able to offer cardiac PET to their patients, making it more convenient to access PET MPI and providing a meaningful impact for clinicians and their patients.”
Kevin O’Neill, CEO of the Pharmaceutical Diagnostics (PDx) segment of GE HealthCare, said, “As the first and only FDA-approved F 18 PET MPI radiotracer for CAD detection, Flyrcado can make a real difference to clinicians and their patients. This is another example of GE HealthCare’s commitment to innovating and investing to shape the future of molecular imaging, increasing diagnostic confidence and addressing unmet patient needs.”
GE HealthCare acquired exclusive global commercialization rights for flurpiridaz F 18 from Lantheus in 2017 and has led the funding and development of the product through to approval. Lantheus collaborated on the development and will also collaborate on commercialization through a joint steering committee. Lantheus is entitled to royalties based on commercial sales milestones.
Flyrcado will be available in initial US markets in early 2025 with expanding availability thereafter.