Extrauterine Adenomyoma
Images
Case Summary
An adult presented with a history of refractory morbid obesity, despite previous gastric banding. The patient’s surgical history also included cholecystectomy, endometrial ablation, and subsequent hysterectomy.
Despite the previous surgery for weight loss, the patient continued to struggle with morbid obesity (BMI: 44-49 range) and ultimately underwent vertical sleeve gastrectomy.
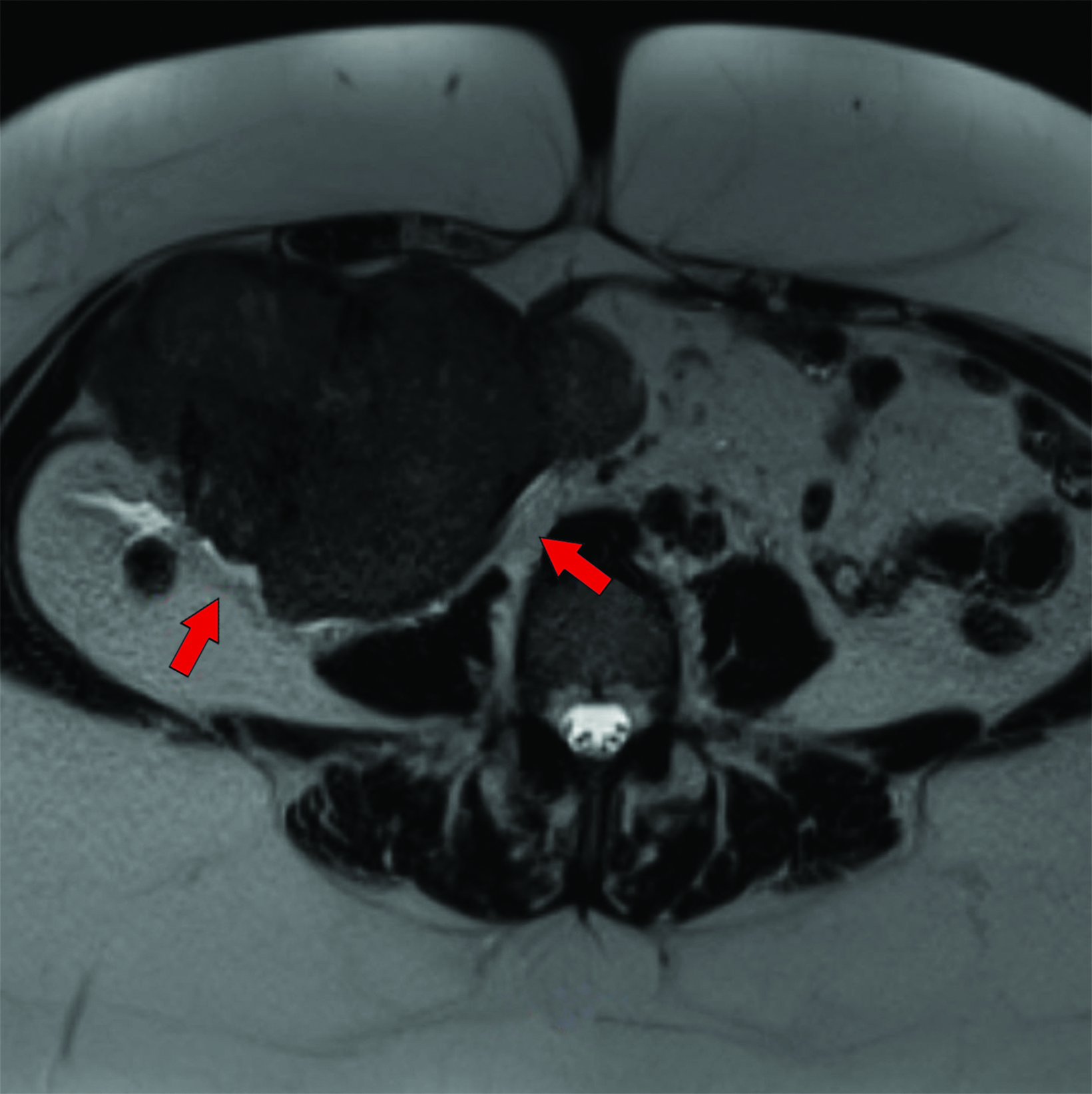
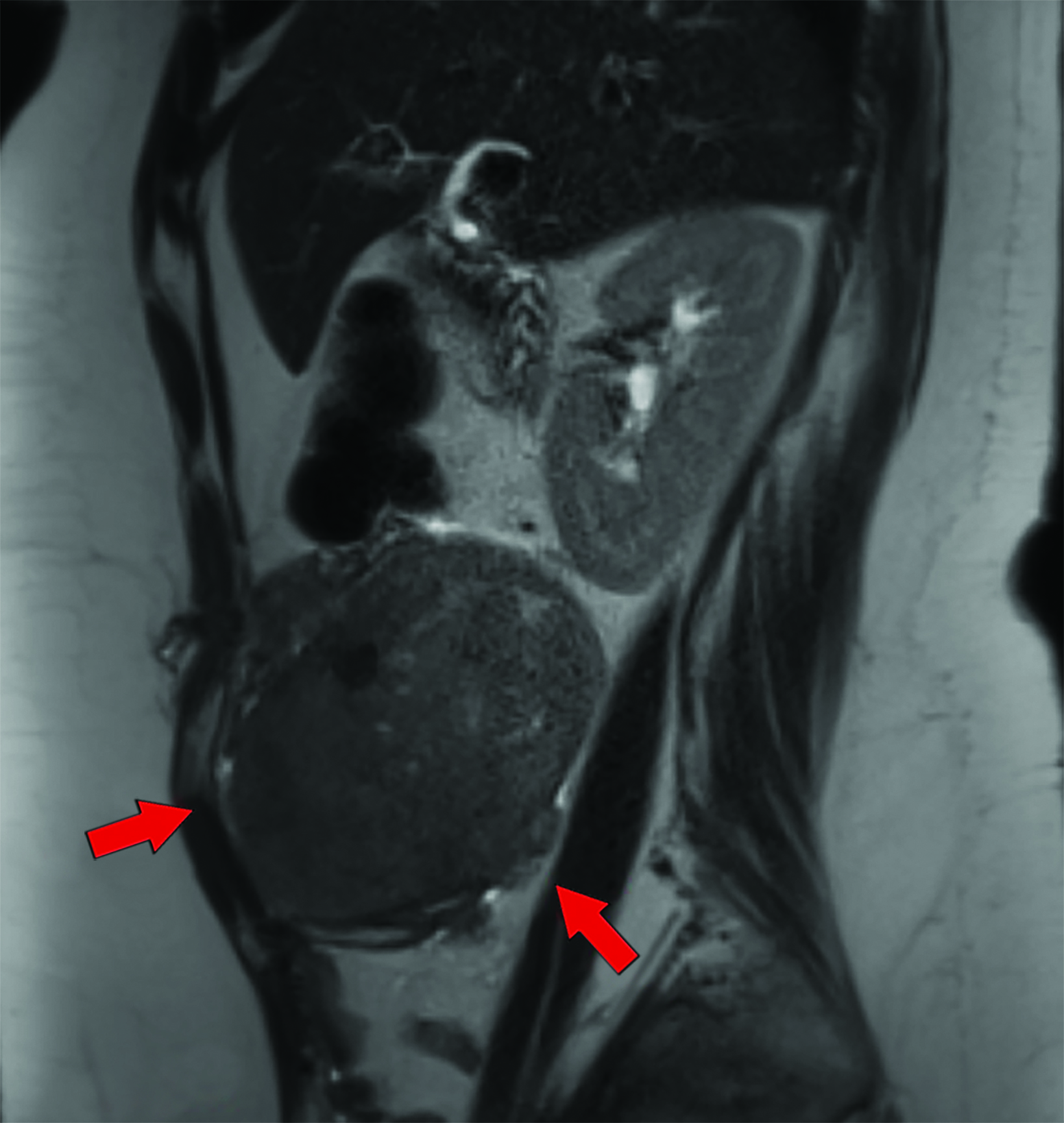
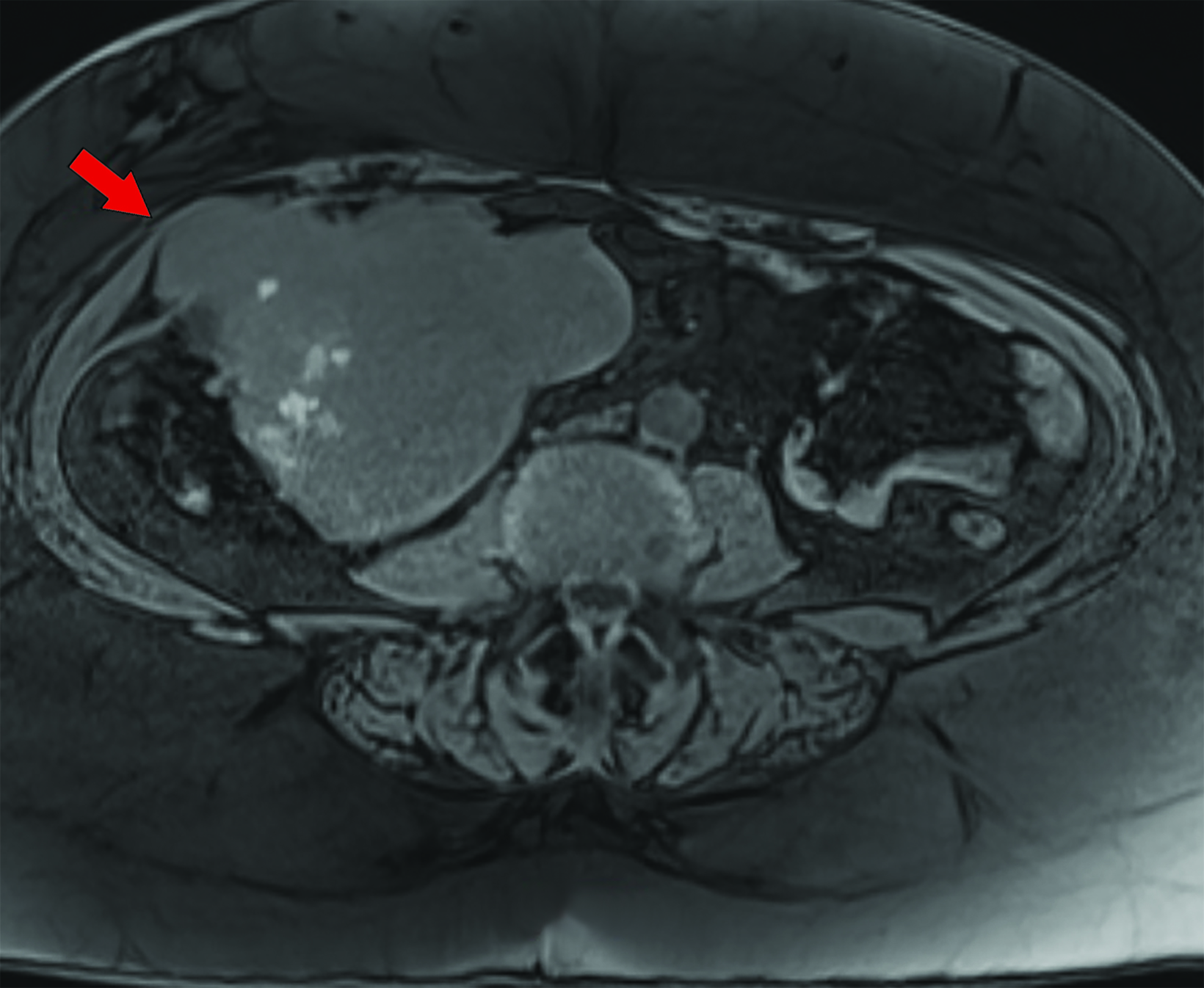
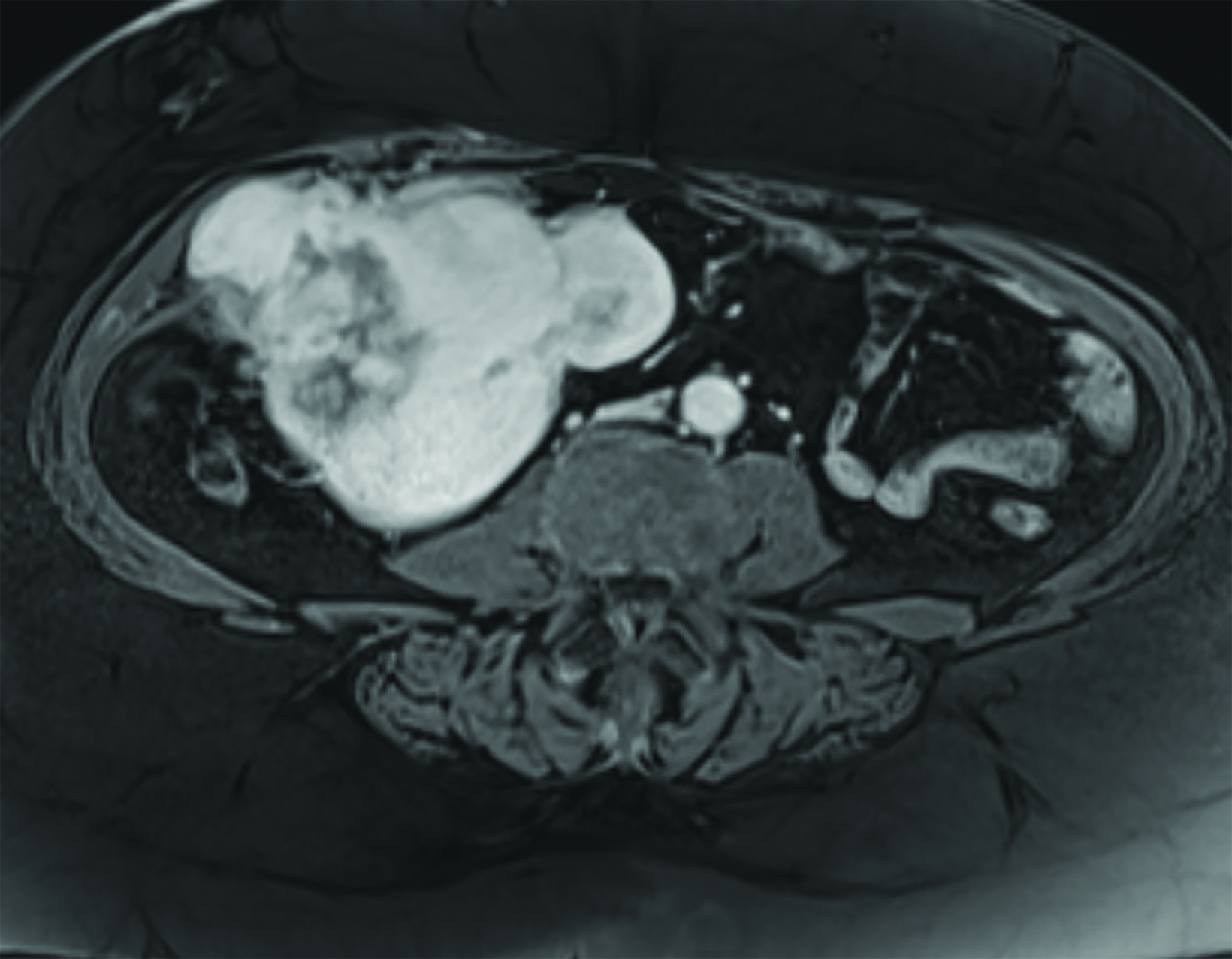
Approximately two weeks postsurgery, the patient complained of abdominal pain and a “pelvic mass” sensation. Evaluation included an abdominopelvic MRI, which demonstrated a right lower quadrant soft-tissue mass protruding through the muscular layers of the anterior abdominal wall. They underwent exploratory laparotomy and tumor resection.
Imaging Findings
Magnetic resonance imaging (MRI) of the abdomen and pelvis revealed a T1 and T2-isointense mass with small foci of intrinsic T1 shortening suggesting areas of blood products (Figures 1-3). Overall, the mass avidly enhanced following gadolinium administration, with some peripheral nonenhancing areas. Chest CT was negative for pulmonary nodules and metastatic disease.
Diagnosis
Extrauterine adenomyoma.
The differential diagnosis of a solid, abdominal wall mass includes sarcoma, abdominal wall desmoid tumor, and endometrioma. Much less likely diagnoses would include solitary lymphoma, metastatic disease, and neurofibroma.
Discussion
Adenomyosis, a common condition, is the presence of endometrial glands and stroma within the myometrium. The condition has an incidence ranging from 20%-60%. Adenomyomas, the localized form, are benign tumors containing smooth muscle cells, and endometrial glands and stroma. They differ from adenomyosis by their distinct borders and being well-circumscribed masses separate from surrounding tissues. The most common locations to find adenomyomas are within the pararectal space, ovary, and broad ligament.1 Infrequently, adenomyomas can occur outside the uterus and, even more infrequently, outside the pelvis. It is often difficult to diagnose adenomyomas preoperatively based on imaging alone, as other benign and malignant entities can have similar imaging appearance.
Currently, there are five proposed theories for the pathophysiology of extrauterine adenomyomas. The first, by Cozzuto et al,2 the first to report extrauterine adenomyoma, suggests they form when a focus of endometriosis undergoes metaplasia to become smooth muscle. The second theory, from Rosai et al in 1982,3 suggests that extra-uterine adenomyomas form from uterine fusion defects, such as a unicornuate uterus, whereby the single horn can detach and implant elsewhere. In 2005, Redman et al4 postulated a sub-coelomic mesenchymal metaplasia theory in which a pleuripotent cell layer beneath the mesothelial layer could differentiate under various hormonal stimuli. The most recent theory, proposed by Batt,5 argues that heterotopic Mullerian-like tissue of embryonic origin could develop within other normal organs. However, a theory proposed by Belmarez et al6 in 2019 fits this patient best. This theory suggests that pelvic seeding can occur during previous surgery, especially when involving morcellation.
The most common complaint in patients with extrauterine adenomyoma is abdominal pain. Occasionally, patients will have abnormal vaginal bleeding or infertility. Of the 34 or so reported cases of extrauterine adenomyomas in the literature, only eight have been extrapelvic,1 and only one is reported to have occurred in the abdominal wall.7 There is scant literature on the radiological appearance, and preoperative diagnosis can be challenging.
Ultrasound, CT, and MRI are the most common modalities used for evaluation; in almost all reported cases, definitive diagnosis was only obtained through histopathologic analysis. On ultrasound these lesions appear as hypoechoic, solid masses with internal vascular flow utilizing color Doppler. On CT and MRI they demonstrate well-defined borders, often lobulated in contour. They are hypointense on T1 and usually isointense to smooth muscle on T2, much like uterine leiomyoma. This signal pattern contrasts with the more common adnexal mass, endometrioma, which is usually bright on T1, owing to the presence of blood products, and dark on T2.
Adenomyomas generally do not demonstrate restricted diffusion. They almost never demonstrate calcifications, and they enhance robustly after administration of IV contrast. MRI is the only modality that demonstrates somewhat reliable findings in differentiating adenomyomas from other adnexal or pelvic masses. The more common form is adenomyosis, seen as diffuse or focal thickening of the T2 hypointense junctional zone. It is common to find punctate foci of high T2 signal scattered throughout the myometrium, indicating islands of ectopic endometrial tissue. When it is a focal mass (ie, an adenomyoma) it may appear as intramyometrial, most often in the body of the uterus. Adenomyomas do not demonstrate the “T2 shading” resulting from the presence of blood degradation products typical of endometriomas.
Surgical resection is almost always curative, although recurrence, and rarely, malignant transformation, have been reported. Clear cell and focal endometriod adenocarcinomas have been reported in extrauterine adenomyomas.8,9
Conclusion
Extrauterine adenomyoma is a benign tumor containing endometrial tissue and smooth muscle cells. It is extremely rare outside of the pelvis. Preoperative diagnosis is often a challenge, as other tumors can have similar appearance. The pathogenesis of these tumors is unclear; however, some evidence implicates hormonal influences, embryologic development errors, and/or mechanical or iatrogenic mechanisms. Definitive diagnosis can only be made by histopathologic analysis.
References
Citation
LJ S.Extrauterine Adenomyoma. Appl Radiol. 2023; (6):46-48.
November 3, 2023