Cortechs.ai Nets FDA Clearance for NeuroQuant 5.0 with Improved ARIA Quantification
Images
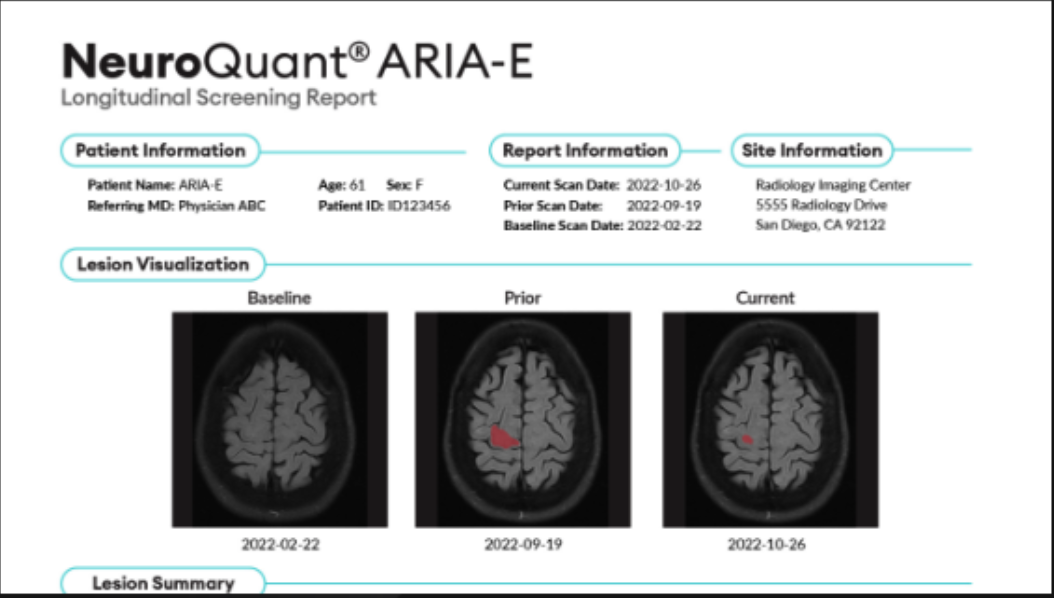
Cortechs.ai received FDA 510(k) clearance for its NeuroQuant 5.0 software, which introduces advanced segmentation and quantification capabilities for MRI lesions associated with T2*GRE and susceptibility weighted imaging (SWI). Notably, the latest crucial advancement empowers radiologists to improve segmentation for Amyloid-Related Imaging Abnormalities (ARIA) in patients undergoing anti-amyloid treatment for Alzheimer’s Disease. NeuroQuant 5.0 improves visualization and calculations of lesions through new enhanced deep-learning based technology, providing critical insights that support more precise and data-driven patient care for managing neurological conditions like Traumatic Brain Injury (TBI), Cerebral Amyloid Angiopathy (CAA), ARIA-E, and ARIA-H.
"Receiving FDA clearance for NeuroQuant 5.0 is a testament to Cortechs.ai’s relentless drive to innovate and lead the industry in imaging-based AI technologies," said Kyle Frye, CEO of Cortechs.ai. "With this release, we are transforming the way radiologists and neurologists approach neurological evaluations, helping to ensure more accurate and timely diagnoses for patients. This marks our 5th FDA approved product, again signifying our pole position as the leader in quantitative AI imaging in both Neurology and Oncology.”
The integration of susceptibility-sensitive MRI sequences in NeuroQuant 5.0 enable unparalleled precision in the segmentation and quantification of even the smallest brain lesions. "Our commitment to staying ahead of the curve and continuously pushing the envelope of what AI can achieve is at the heart of NeuroQuant 5.0," said Nate White, CTO of Cortechs.ai. "This software equips clinicians with cutting-edge tools to better detect and quantify brain lesions with greater accuracy and precision, aiding in the management of complex conditions such as TBI and Alzheimer."
With NeuroQuant 5.0, the company reinforces its commitment to empowering clinicians with advanced, automated tools that enhance the evaluation of brain pathologies and elevate patient care to new heights.