Bracco Announces U.S. FDA Approval of 20-Vial Configuration of LUMASON
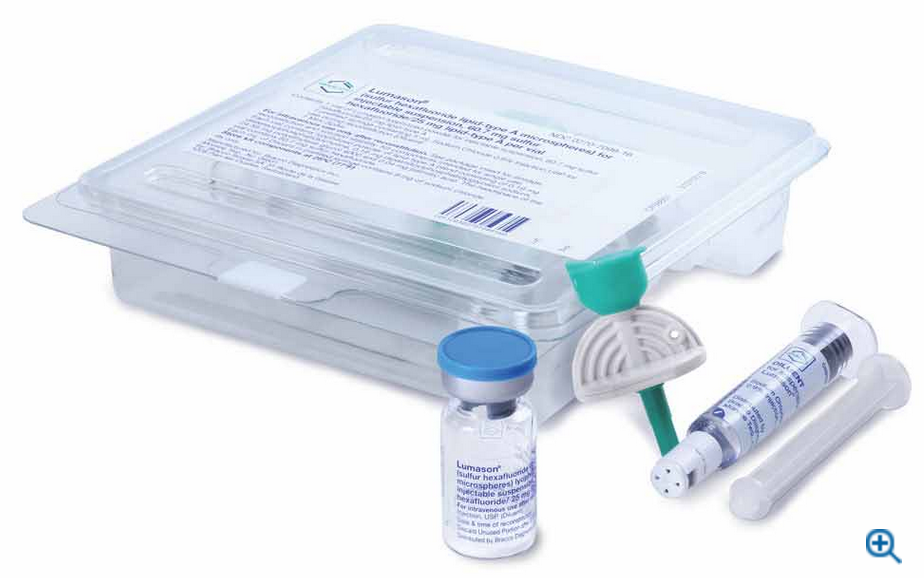 Bracco Diagnostics Inc., the US subsidiary of Bracco Imaging S.p.A., announced today that its ultrasound enhancing agent (UEA) LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use, received FDA approval on a new 20-vial pack configuration. This new product configuration provides customers with a green packaging alternative, increased storage space efficiency, and more SKU options.
Bracco Diagnostics Inc., the US subsidiary of Bracco Imaging S.p.A., announced today that its ultrasound enhancing agent (UEA) LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use, received FDA approval on a new 20-vial pack configuration. This new product configuration provides customers with a green packaging alternative, increased storage space efficiency, and more SKU options.
“Bracco is proud to launch this new configuration of LUMASON UEA while still featuring our needleless, air sterile filtration Mini-Spike, protecting you and your patients1,” said Cosimo De Pinto, Vice President of Sales and Marketing at Bracco Diagnostics Inc. “Whether you are a nurse in the ICU or a sonographer in a high-volume lab where storage is limited and productivity is key, we are committed to providing options that meet our customers' needs.”
LUMASON UEA, known globally as SonoVue, has a proven safety profile and is the only agent that has multiple indications for adult and pediatric patients2. This new configuration features 20 vials of LUMASON UEA, each containing 25 mg of lipid-type A lyophilized powder and 60.7 mg sulfur hexafluoride headspace, and a corresponding Mini-Spike for each vial2.
Marketed since 2001 and available in more than 40 countries3, LUMASON UEA is comprised of gas-filled microspheres that reflect the sound waves to enhance the echogenicity of the blood or urine, which results in an improvement in the diagnostic quality of the ultrasound images4. The agent does not require refrigeration or mechanical agitation.
See full Prescribing Information for LUMASON ultrasound contrast agent including boxed warning at https://www.braccoimaging.com/us-en/products/contrast-enhanced-ultrasound/lumason.
- Braun Product Specification: Product Group: Mini-Spike, Data on File, 2012. pp. 2-4.
- LUMASON®(sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; December 2020.
- Data on file. Bracco Diagnostics Inc. based on Global Contrast-Enhanced Ultrasound (CEUS) DOLAN, February 2021.
- Data on file. Bracco Diagnostics Inc. May 2019. Geneva data 2019.