A New Era for Quantitative Brain MRI Analysis
Images
Radiology has been among the early adopters of advances in technology, whether they are new scanner technologies, novel imaging sequences, machine-assisted acquisition and reconstruction techniques, or new tools for image interrogation. Technological advances in medicine over the last decade have been driven largely by artificial intelligence (AI), and radiology is leading the pack in the number of AI-based medical devices cleared by the US Food and Drug Administration.1
AI-fueled solutions already impact imaging at numerous points, ranging from scheduling through reporting. Deep learning-based tools improve the quantitative analysis of studies, triage urgent examinations for prioritized attention, and are beginning to emerge with branded FDA clearance for true diagnostic support.
In the coming years and decades, the need for imaging will only grow, driven in part by the nation ’s aging population. In addition, with the availability of new disease-modifying therapies, there is an urgent need for AI-based assistive solutions that can quantify health — particularly brain health — and monitor therapeutic progress in patients. The July 2023 FDA approval of Leqembi® (developed by Eisai and Biogen), the only disease-modifying treatment shown to reduce the rate of cognitive and functional decline in Alzheimer disease, offers potentially life-changing impacts for those with dementia and their loved ones.2 Physicians, healthcare systems, and payers will need to prepare for the numerous implications.
Leqembi® is expected to substantially increase the need for MRI capacity, given the large population of people expected to require therapy as well as those seeking imaging to assess early cognitive impairment. Per FDA guidance, treatment with Leqembi® is appropriate only in patients with early disease, putting a premium on early identification. However, at the moment, Alzheimer disease is diagnosed three years after the appearance of symptoms in 80% of patients.3 Hence, AI-driven quantitative MRI analysis tools have the potential to support differentiating Alzheimer disease from other dementia subtypes and normal aging.
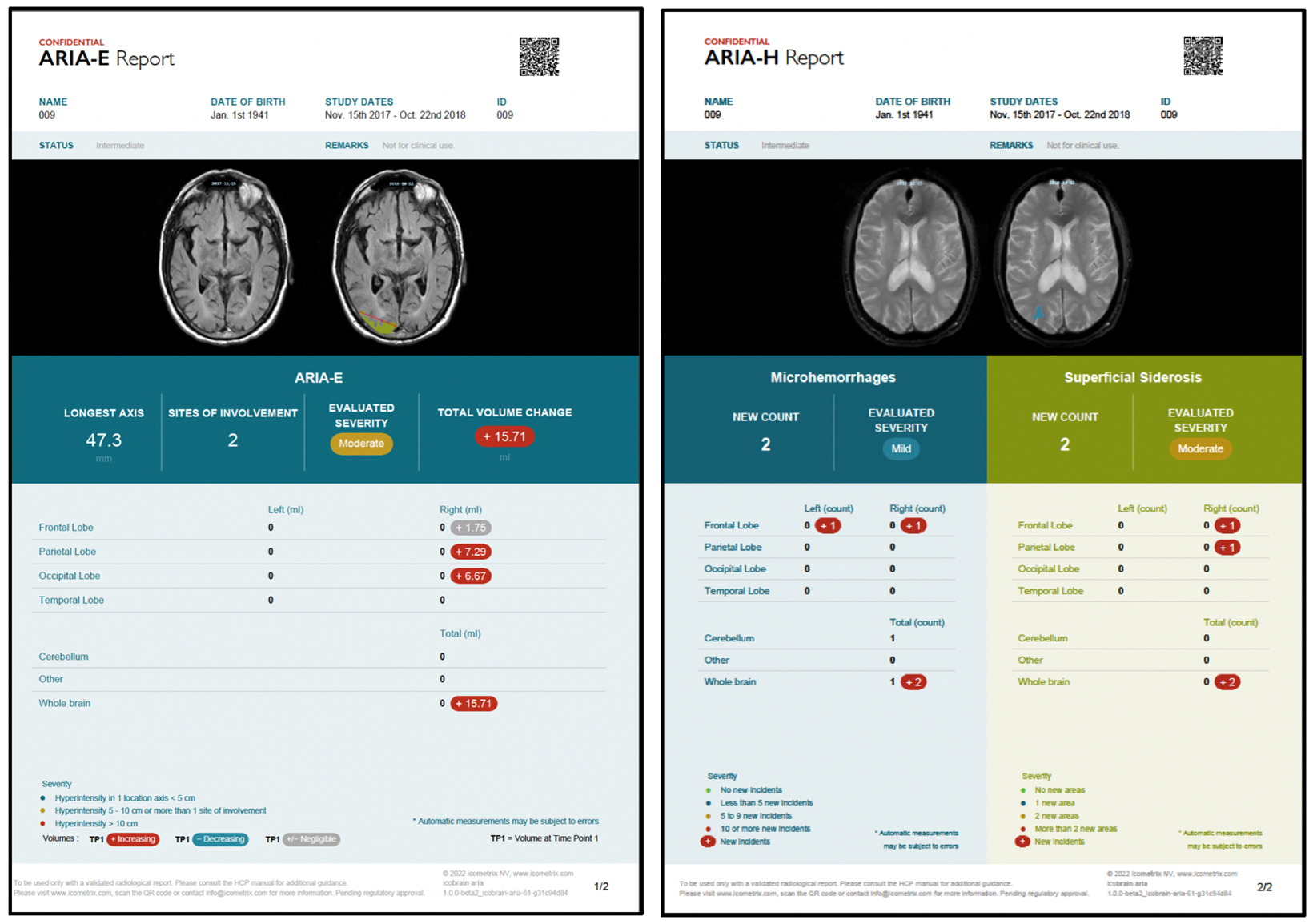
In addition, amyloid targeting therapies are associated with amyloid related imaging abnormalities (ARIA), such as edema and sulcal effusions termed ARIA-E, as well as microhemorrhages and siderosis referred to as ARIA-H. As a result, the FDA currently mandates a recent pre-treatment brain MRI as well as surveillance MRI scans prior to the fifth, seventh, and fourteenth biweekly Leqembi® infusions.2
As ARIA reading may be new to many radiologists, physician education would be beneficial. Findings may be subtle and thus, reliable and consistent disease identification and quantification is required. For example, radiologists will need to quantify the number of new microhemorrhages and the longest axis of an edema focus.4 Hence, there is an important role for AI algorithms that can assist in ARIA reading and severity assessment. Because a standardized, quantitative, and accurate assessment of ARIA is critical for treatment safety and efficacy, AI solutions of the highest standard of validation and accuracy will have the best chance of routine adoption. In this context, several AI tools specifically for ARIA monitoring are being developed and/or under active FDA review (Figure).
A last hurdle standing in the way of day-to-day adoption of quantitative imaging AI solutions, is a sustainable business model for users and providers. As we experienced with 3D visualization tools two decades ago, a dedicated current procedural terminology (CPT) billing code is needed to elevate adoption to standard of care. Today, we indeed cannot imagine not using a 3D visualization solution for modalities like CT angiography. Fortunately, just as this wave of brain scans requiring evaluation begins flooding healthcare systems, a new CPT III code for MRI brain scan quantification has recently become available. Through the efforts of icometrix, a company which offers the icobrain™ suite of solutions for brain MRI and CT, the American Medical Association on July 1, 2023, issued CPT III codes 0865T and 0866T. The widespread use of these codes for billing is the next critical step toward routine and reimbursement.
In summary, radiologists should anticipate a wave of brain MRI study requests in light of new therapeutic options and the imminent arrival of reimbursement for AI-based quantification. The time is now for radiologists to adopt these AI solutions for next-level clinical care.
References
Citation
W VH, LN T.A New Era for Quantitative Brain MRI Analysis. Appl Radiol. 2023; (5):24-25.
September 12, 2023