Phase 3 Clarity AD Study Shows Reduction in Amyloid-Beta Pathology and Biomarker Changes
Images
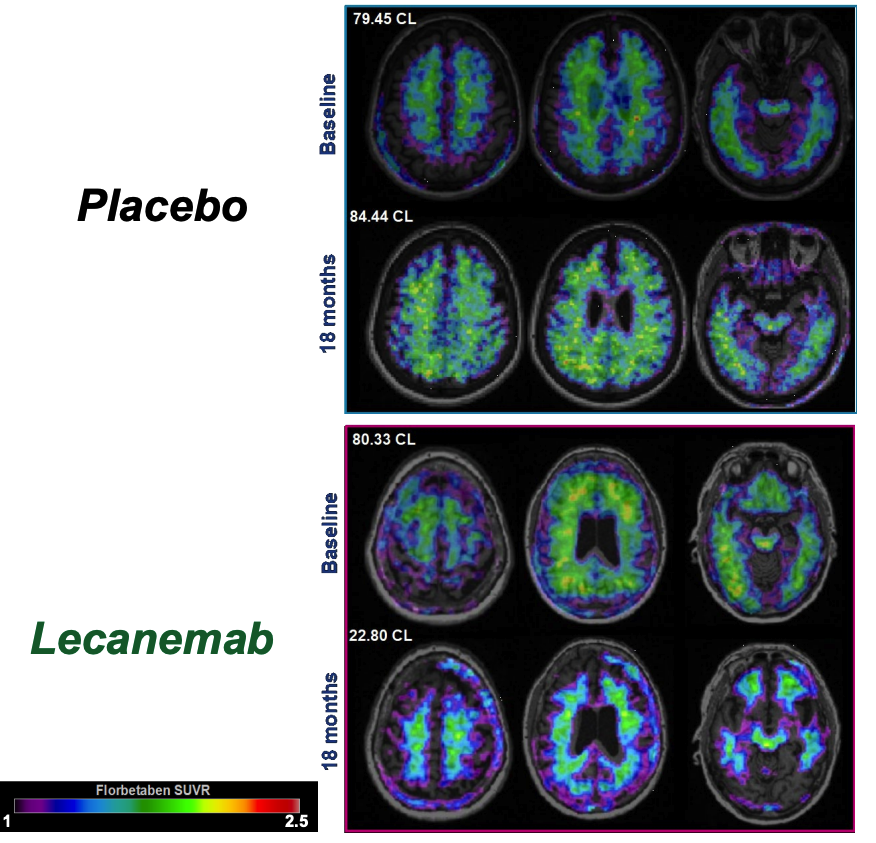
Eisai Co., Ltd. and Biogen Inc. announced the results of a detailed analysis of the Phase 3 Clarity AD study demonstrated that lecanemab-irmb (generic name, US brand name: LEQEMBI) treatment showed reductions in amyloid-beta (Aβ) pathology and downstream biomarker changes. This analysis, and the latest findings on the lecanemab subcutaneous (SC) formulation currently under development, were presented at the Alzheimer's Association International Conference (AAIC) 2023.
Clarity AD was a global confirmatory Phase 3 placebo-controlled, double-blind, parallel-group, randomized study in 1,795 people with early AD (lecanemab group: 10 mg/kg bi-weekly IV treatment: 898, placebo group: 897). Lecanemab met the primary endpoint (change from baseline at 18 months on the global cognitive and functional scale, Clinical Dementia Rating-Sum of Boxes [CDR-SB]) and all key secondary endpoints with statistically significant results.
In addition to the key secondary endpoint of lecanemab's effect on amyloid accumulation in the brain as measured by amyloid positron emission tomography (PET), the Clarity AD study also measured multiple A/T/N+ (amyloid, tau, neurodegeneration) biomarkers involved in the pathophysiology of AD, such as amyloid (Aβ1-42 in CSF, Aβ42/40 ratio in plasma), tau (p-Tau181 in cerebral spinal fluid [CSF] and plasma), neurodegeneration (total tau [t-tau] in CSF and neurofilament light [NfL] in CSF and plasma), astrocyte activation (plasma GFAP: glial fibrillary acidic protein) and synaptic dysfunction (neurogranin in CSF).
An increase in plasma Aβ42/40 ratio was observed with lecanemab compared to placebo (adjusted mean change from baseline of lecanemab: 0.008, placebo: 0.001, p<0.0001). A reduction in plasma p-Tau181 was observed with lecanemab compared to placebo (adjusted mean change from baseline of lecanemab: -0.575 pg/mL, placebo: 0.201 pg/mL,
p<0.0001). The other biomarkers also improved after treatment with lecanemab. These outcomes suggested lecanemab impacts A/T/N+ biomarkers involved in the AD pathophysiology and exerts biological effects that demonstrate slowing of disease progression.
Baseline characteristics and initial results were presented from the tau PET substudy of Clarity AD. Lecanemab administration slowed the accumulation of tau pathology in the temporal lobe. Additionally, lecanemab administration showed a clinical effect in the overall population of the tau PET substudy, and a large effect size was observed in the low tau population defined in this presentation, which represents the early phase of AD. Using the MK6240 tau PET probe, tau accumulation in the brain was defined as low tau accumulation group (MK6240 cutoff value <1.06, 141 subjects), intermediate accumulation group (MK6240 cutoff value between 1.06 and 2.91, 191 subjects), and high accumulation group (MK6240 cutoff value >2.91, 10 subjects).
In an exposure/bioavailability and modeling study comparing intravenous (IV) and subcutaneous (SC) dosing of lecanemab, the bioavailability of SC dosing of lecanemab was shown to be approximately 50% of that of IV dosing. Further analysis using the PK/PD model showed that a fixed lecanemab SC dose of 720 mg administered weekly may potentially result in comparable exposure (area under the curve [AUC]) and efficacy as measured by reduction in amyloid PET SUVr to 10 mg/kg IV dose administered bi-weekly. Models developed with data following IV administration show that amyloid-related imaging abnormalities with edema/effusion (ARIA-E) are related to concentrations of lecanemab in the blood, with maximum blood concentrations being the best predictor of ARIA-E. Because SC dosing will have lower maximum blood concentrations than IV dosing, SC dosing is predicted to have a lower incidence of ARIA-E, if the relationship is the same for SC dosing.