Radiological Case of the Month
Images
Prepared by Sonal Krishan, MD; Ravi Solanki, MD; Sumer Kumar Sethi, MBBS, Department of Radiodiagnosis, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India.
CASE SUMMARY
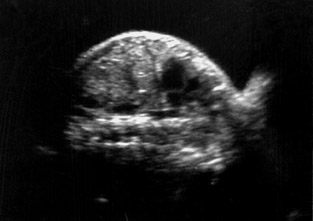
A pregnant woman (gravida 2 para 1 with 1 live issue) was referred to our department for routine obstetrical ultrasound. There was no family history of any birth defects. Her first child was healthy. According to dates, the fetus was 31 weeks gestation. On ultrasound scanning, she had a single live intrauterine fetus with cephalic presentation of 32 ± 3 weeks by fetal biometry (Figure 1).
DIAGNOSIS
Congenital esophageal atresia (CEA)
IMAGING FINDINGS
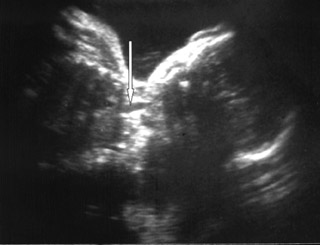
On ultrasound, amniotic fluid appeared to be a higher volume than expected for gestational age. Fetal stomach bubble was not visualized during the 45-minute sonographic examination (Figure 1). Fetal skull, spine, bilateral kidneys, and urinary bladder were normal. All four fetal limbs were identified, and there was no evidence of radial atresia (not shown). On a coronal ultrasound scan of the fetal neck, a transient anechoic tubular area was observed in the midline in the fetal neck, which seemed to distend during fetal swallowing (Figure 2).
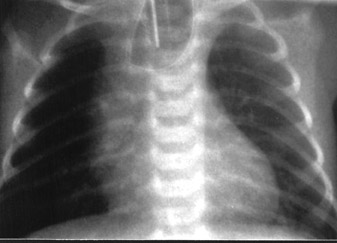
The patient had a spontaneous delivery at gestational week 36. The baby was diagnosed as having esophageal atresia (EA) with the "coil-up sign" within the dilated blind-ending esophagus on the plain postnatal chest X-ray (Figure 3) and required immediate surgical treatment for respiratory distress. The operation was uneventful, and the patient was discharged in satisfactory condition. At 8-month follow-up, the child appeared to be developing normally and could be fed regularly.
DISCUSSION
Prenatal ultrasonographic suspicion of EA is usually based on the presence of both polyhydramnios and a fetal stomach that is either absent or shows reduced filling. 1 The ultrasound diagnosis is difficult because there is often a tracheoesophageal fistula that allows passage of fluids and consequently causes stomach distension. Even in cases of atresia without fistula, a moderate distension may occur as a consequence of gastric secretion. 2 None of these criteria is definite. Furthermore, when the diagnosis is based on these two signs, the outcome cannot be predicted before birth. In a study by Stringer et al 3 the positive predictive value of an absent stomach bubble and polyhydramnios was 56%. There are numerous other causes than EA of an absent or small fetal bubble. These are largely related to impaired fetal swallowing due to mechanical obstruction, facial clefts, central nervous system abnormalities and neuromuscular syndromes.
Prenatal diagnosis of EA enables parents to be prepared for the birth and treatment of their affected child, permits prompt neonatal management, and may lead to earlier identification of associated anomalies (eg, VACTERL association). Important clues include radial atresia, vertebral defects, and renal anamolies. 4
Eyheremendy et al 5 originally reported 2 cases of EA with antenatal ultrasonographic findings of an alternating filling and emptying of a large proximal esophagus. Satoh et al 6 extended these preliminary findings to all cases with both polyhydramnios and a small or absent stomach bubble. Eight of 10 such cases having an anechoic area in utero were confirmed postnatally to have CEA. These findings were consistent with our case. Based on postnatal findings, these 8 cases with an anechoic area in the neck had either Gross type A or type C CEA. Our case had type A CEA. Both of these types have a proximal pouch. The anechoic area in the neck presumably indicates transient fluid pooling in the proximal pouch of the esophagus derived from the swallowing of amniotic fluid. Kalche et al 7 found that a pouch with a base situated within the thorax was always associated with distal tracheoesophageal fistula and primary esophageal repair. They also illustrated that a neck pouch was more likely to be associated with a long atretic gap. 7
For optimal visualization, the fetal neck should be imaged in a coronal plane. The ultrasonographic transducer is maneuvered so that hypopharynx, larynx, and trachea are visible in the same image. As the transducer is moved posteriorly, the trachea disappears from view and the esophageal region becomes visible. A cystic pouch is discernable in the region of the esophagus and is seen periodically to fill and empty in accordance with fetal swallowing. 7 Shulman et al 8 state that a systematic multiplanar imaging of fetal upper body is a prerequisite for diagnosing EA. A sagittal view is recommended for visualizing a low-level pouch, and coronal views are optimal for revealing a high pouch. 8
However, not all fetuses with CEA will have a positive neck pouch sign. In a case reported by D'Elia et al, 9 no fetal neck pouch was detected although the patient had type C CEA on postnatal follow-up. Therefore, further studies are necessary to clarify the independent diagnostic value of the fetal neck pouch sign. In addition, further investigations are needed to elucidate the diagnostic value of this anechoic area in the antenatal diagnosis of type B, D, and E CEA, which have a fistula between the proximal pouch of the esophagus and either the trachea or the distal pouch. It has also been suggested that CEA may be associated with abnormal esophageal peristalsis. 9 Fetal neck pouch sign may not be discernable in such cases.
Earliest reported suspicion of EA was raised in the 22nd week of pregnancy in the presence of a small stomach bubble associated with a persistent left superior vena cava. 10 Kalache et al 7 have postulated that difficulty in detecting the pouch sign before the 26th week, even in the most common type of EA (Type C), is due to the fetus's inability to generate sufficient pressure when swallowing to permit dilatation of blind-ending esophagus.
Any anechoic area in the fetal neck must be differentiated from other diseases, such as cervical cystic hygroma, cystic teratoma, or thyroglossal cyst. The anechoic area, however, can be easily differentiated from these anomalies by its transient appearance, as well as its position.
Trisomy 18 was present in almost half of the fetuses in the series reported by Stringer et al. 3 If a fetal sonogram suggests the presence of EA, karyotyping should also be considered, particularly if additional anomalies are detected. 3 However, karyotyping was not done in our case.
Fetal magnetic resonance imaging (MRI) has recently been added to the armamentarium of radiologists, guiding prenatal diagnosis and management. Langer et al 11 evaluated the accuracy of fetal sonography followed by MRI for the diagnosis of EA. Fetuses considered to be at risk for EA, based on detailed obstetric sonography, underwent MRI. They found that sensitivity of MRI was 100% and specificity was 80% in prenatal diagnosis of EA. They concluded that MRI appears to be accurate for establishing or ruling out a prenatal diagnosis of EA and should be considered in fetuses that are at high risk based on ultrasonic findings. 11
CONCLUSION
Prognosis of a fetus with a suspected EA must be guarded and is influenced by many factors, including the presence of other fetal anomalies, results of fetal karyotyping, and diagnostic accuracy of prenatal ultrasonography. Although it is difficult to determine the individual prognosis, the high probability of fetal neck pouch sign may assist in deciding perinatal management. Further, fetal MRI could be carried out in suspected cases to confirm ultrasonographic findings. Although recent ultrasound techniques have improved their diagnostic value for EA, further studies are required to prevent the many false positives and false negatives that may cause difficulty in the proper counseling of these patients.
Related Articles
Citation
Radiological Case of the Month. Appl Radiol.
January 18, 2005