Shoulder Injury Related to Vaccine Administration: Isolated Teres Minor Tear
CASE SUMMARY
A 59-year-old female with a weight of 153 lbs. (69 kg) and no significant medical history presented with acute left shoulder pain and limited range of motion that began fewer than 24 hours after receiving the tetanus and diphtheria (Td) vaccine for routine prophylaxis. The patient denied fever, prior shoulder pain, trauma, excessive lifting, pulling or exercising. On physical exam, the patient experienced limited range of motion, pain with active and passive movement, and tenderness over the deltoid tuberosity. The patient’s reflexes, strength, and sensation were intact. After minimal relief with nonsteroidal anti-inflammatory drugs (NSAIDs), nonenhanced magnetic resonance imaging (MRI) of the left humerus was obtained.
An MRI performed 6 weeks from the start of symptoms demonstrated a high-grade partial-thickness tear of the teres minor tendon. In lieu of these findings, an orthopedics consult was recommended. At the time of the orthopedics visit, 7 weeks since the onset of injury, the patient’s symptoms had drastically improved. The patient reported minimal pain with certain motions, such as getting dressed. The physical exam was positive for minimal tenderness at the deltoid insertion site. Reflexes, sensation, and strength were maintained. Given the improvement in symptoms, conservative management was recommended. The patient was started on a course of NSAIDs and referred to physical therapy for rotator cuff stretching and strengthening. At 2-month follow-up, the patient was back to her baseline without shoulder pain.
IMAGING FINDINGS
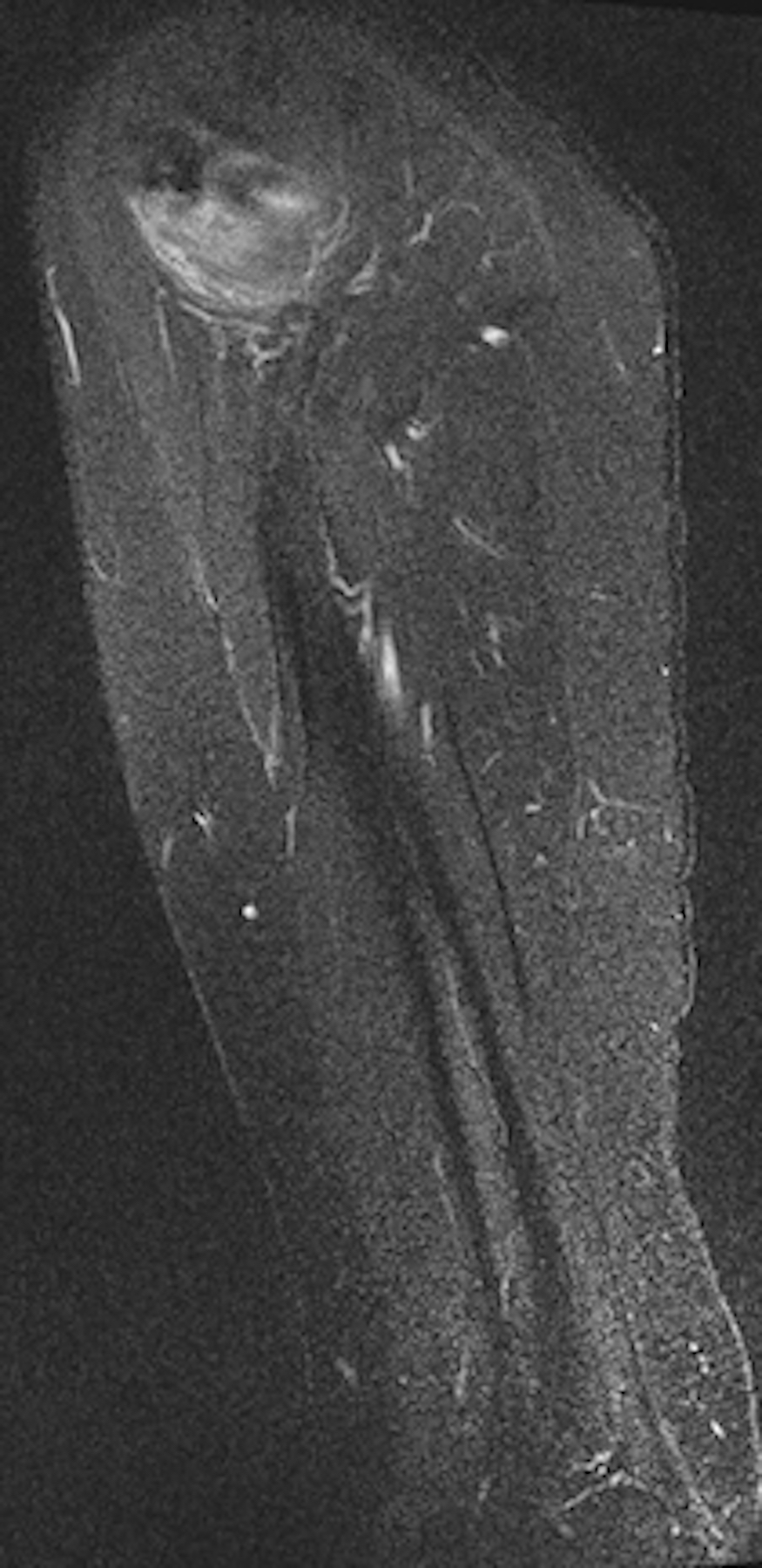
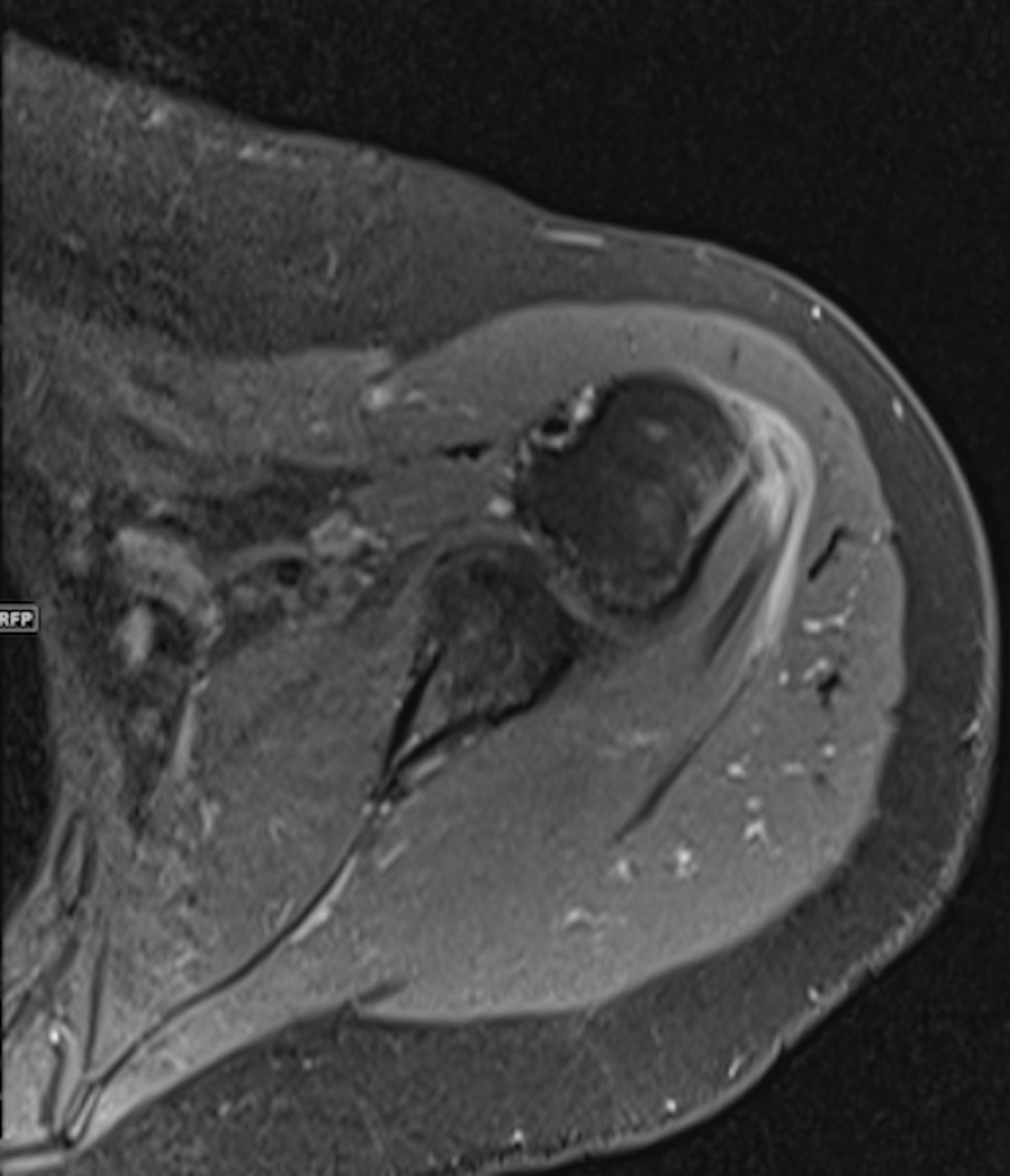
An MRI performed 6 weeks from the start of symptoms demonstrated extensive edema surrounding the teres minor myotendinous junction and tendon (Figure 1). There was an associated high-grade partial-thickness tear of the teres minor tendon (Figure 2). Accompanying reactive humeral subcortical edema at the teres minor tendon insertion was also noted (Figure 2).
DIAGNOSIS
Isolated teres minor tear, a shoulder injury related to vaccine administration
DISCUSSION
Transient pain, erythema, and induration are commonly experienced symptoms following vaccine administration in the shoulder. Infrequently, however, vaccines may result in an entity documented as shoulder injury related to vaccine administration (SIRVA) by the National Vaccine Injury Compensation Program. Symptoms of SIRVA include persistent shoulder and arm pain, restricted motion, bursitis, and brachial palsies. Few reported cases in the literature define the imaging findings related to this entity. To the best of our knowledge, we report the first case of an isolated teres minor rotator cuff tear following administration of the Td vaccine.
Temporary shoulder pain following vaccine administration is a commonly encountered phenomenon. Significant subcutaneous soft tissue swelling in the injected arm can be seen on ultrasound. These findings are due to a local inflammatory response following immunizations. However, prolonged shoulder pain and restricted motion after vaccine administration may be related to SIRVA, a rare entity described as a complication of incorrect vaccine administration, oftentimes at an injection site located too highly on the shoulder. The result is an immune-mediated inflammatory reaction locally within the shoulder.1 The prolonged immune mediated inflammatory response appreciated in SIRVA is an example of a type III hypersensitivity (Arthus) reaction.2 This phenomenon is due to the body’s reaction to previously residing antibodies (a previous Td injection in our patient’s case) against the newly injected antigens. In our patient, the humeral head edema and teres minor tear depicted on the MRI reflects sequela of direct needle impact and prolonged immune response.
Fewer than 30 cases related to SIRVA are reported in the literature. The largest study, by Atanasoff et al, incorporated 13 cases in which patients received a vaccination against influenza, Td, Tdap, or human papillomavirus and developed prolonged shoulder pain with associated bursitis, tendonitis, and/or rotator cuff tears as seen on MRI.1 Cases of SIRVA have also been reported following other vaccines including pneumococcal and hepatitis.3,4
Nearly all the reported cases demonstrate similar radiographic findings related to SIRVA, including subacrominal and subdeltoid bursitis, tendonitis, supraspinatus and infraspinatus rotator cuff tears, and bone marrow edema. Only one report involving teres minor pathology has been described in the literature.5 In this study, a 60-year-old female experienced prolonged left shoulder pain after receiving the Tdap vaccine. Subsequent MRI revealed a 0.6 x 0.8 cm partial thickness tear of the infraspinatous tendon extending inferiorly to involve the superior portion of the teres minor tendon.
Isolated tears of the teres minor tendon are rare. They occur more commonly in the setting of traumatic posterior shoulder dislocation or in combination with supraspinatus and infraspinatus tendon tears.6,7 We report a previously undescribed case of an isolated teres minor rotator cuff tear following administration of the Td vaccine. The intent of this study is not to foster vaccine hesitancy but to broaden the differential diagnosis of prolonged shoulder pain in the context of recent immunization and to discuss technique.
Our patient experienced signs and symptoms consistent with previously reported cases of SIRVA. The patient developed persistent shoulder pain and limited range of motion in the setting of recent vaccination and no past medical history of shoulder dysfunction. Furthermore, her symptoms began to improve 6 weeks after onset. These findings are similar to those described by Dumonde et al, who proved that antigen injected within synovial tissues resulted in a prolonged immune response lasting 6 weeks, after which symptoms began to improve.8
A decision on needle length and injection site must be made for each person on the basis of the volume of vaccine to be administered, injection technique, size of the deltoid muscle, thickness of adipose tissue at the injection site, and depth below the muscle surface into which the material is to be injected. Most vaccines are administered via an intramuscular route into the deltoid or anterolateral aspect of the thigh. The deltoid muscle is recommended for use with small volume injections, for example vaccinations. This enhances the immunogenicity of the vaccine and minimizes adverse reactions at the injection site. For intramuscular injections, the needle should be long enough to reach the muscle mass and prevent the vaccine from seeping into the subcutaneous tissues, but not so long as to involve the underlying nerves, blood vessels, or osseous structures. Intramuscular injections should be administered at a 90-degree angle to the deltoid muscle using a 22-25-gauge needle. According to the Centers for Disease Prevention and Control (CDC), for men and women <130 lbs (<60 kg), a 5/8-1 inch needle is sufficient to ensure intramuscular injection into the deltoid muscle. For men and women who weigh 130-152 lbs (60-70 kg), a 1-inch needle is adequate.9
The potential risks of under-penetration of vaccines are well known, including local dermatologic reactions and decreased immunogenicity. However, less well known are the adverse effects related to over-penetration and risk of injury deep to the deltoid muscle using needle lengths recommended by the CDC. Lippert et al demonstrated that patients who receive vaccinations in the shoulder utilizing the CDC’s recommended 5/8, 7/8, and 1-inch needle lengths would experience 11% (16 of 150), 55% (83 of 150), and 61% (92 of 150) risk of over-penetration, respectively.10 The authors suggested a weight based vaccination model, wherein a 1/2 inch needle be used in females ≤70 kg and males ≤75 kg, 5/8-inch needle in females 70-115 kg and males 75-140 kg, and 7/8-inch or longer needle in females 115 kg or more and males 140 kg or more. Furthermore, the authors claimed that these weight-based needle lengths could potentially allow for a 0% over-penetration and 10% under-penetration rate. Bodor et al used ultrasound to determine that the sub-deltoid bursa extended 3-6 cm beyond the lateral border of the acromion and 0.8-1.6 cm deep to the skin surface.3 The authors concluded that the subdeltoid bursa is easily accessible by a 1-inch needle thereby making it a potential site of injury during vaccine administration.
Given these recommendations, our patient at a weight of 153 lbs (69 kg) would require a 1/2-inch needle, significantly decreased in length as compared to that recommended by the CDC, thereby, potentially preventing SIRVA from occurring. However, in this patient with a small muscle mass, a 1-inch needle length was employed. Hence, it is very plausible that there was over-penetration of the vaccine through the deltoid into the teres minor tendon at its insertion onto the posterior aspect of the greater tuberosity of the humerus with resultant partial tear of the teres minor tendon.
CONCLUSION
Prolonged shoulder pain and restricted movement following vaccine administration should prompt clinicians to rule out SIRVA as a culprit. It is imperative when administering vaccines to remain within the deltoid muscle and avoid over-penetration into the rotator cuff tendons as this may lead to SIRVA. Lastly, SIRVA should be considered as a plausible cause when faced with isolated teres minor tendon pathology in the appropriate clinical setting.
REFERENCES
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010; 28(51):8049-8052.
- Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007; 137:Suppl 1, S46-50.
- Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007; 25(4):585-587.
- Degreef I, Debeer P. Post-vaccination frozen shoulder syndrome. Report of 3 cases. Acta Chir Belgi. 2012; 112(6):447-449.
- Egol AJ, Broder KJ, Strauss EJ. Vaccination induced rotator cuff tear: A case report. Case Reports in Internal Medicine. 2015; 2(3):18-21.
- Hottya GA, Tirman PF, Bost FW, Montgomery WH, Wolf EM, Genant HK. Tear of the posterior shoulder stabilizers after posterior dislocation: MR imaging and MR arthrographic findings with arthroscopic correlation. AJR Am J Roentgenol. 1998; 171(3):763-768.
- Lee SW, Park SE, Park MG, Ji JH. Arthroscopic Treatment of Isolated Teres Minor Tendon Tear: A Case Report. Clinics in Shoulder and Elbow. 2015; 18(3):159-161.
- Dumonde DC, Glynn LE. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962; 43(4):373-383.
- Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP).
- Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008; 122(3):556-563.
Prepared by Dr. Bansal while a PGY-4 Radiology Resident and Dr. Di Lorenzo while the section head of Musculosketal Radiology, at MetroHealth Medical Center/Case Western Reserve University, Cleveland, OH.
