What Does CMS Dose Compliance in Radiology Mean for You?
Background
First, the Short Answer
The Centers for Medicare & Medicaid Services (CMS) has slated voluntary public reporting of metrics related to radiation dose and image quality in calendar years 2025 and 2026, with mandatory reporting beginning in 2027. While the financial incentives or penalties tied to performance remain to be defined, institutions should be preparing for the inclusion of such compliance and reporting in broader CMS quality and payment programs.1 These temporal horizons are clearly moving toward us, so any radiology practice would be wise to proactively consider the installation or refinement of appropriate software and workflow to be compliant.
Wait, How Did We Get Here? (The Longer Answer)
Computed tomography (CT) radiation doses, even when administered for identical clinical indications, can vary dramatically across patients, institutions, and scanner models, a variability that has been historically driven more by practice preferences than by consistent evidence-based thresholds. Although the American College of Radiology’s Dose Index Registry enables benchmarking of local dose performance against regional and national aggregates, it offers no mandatory dose limits or formal guidance on what constitutes an excessive dose.2 Indeed, a phantom-based study of standard body CT protocols revealed dose exposures that differed significantly depending on the specific scanner used, indicating that even patients of similar size may receive widely disparate radiation levels for the same exam.3
While quantifying the effects of ionizing radiation from CT involves extrapolation and some uncertainty, the consensus continues to be that it carries a nontrivial risk of inducing malignancy, particularly when cumulative exposures are considered over a lifetime. The National Academies’ BEIR VII Phase 2 report remains the authoritative source on low-level radiation risk, estimating increased cancer incidence in exposed populations based on epidemiologic and mechanistic data.4 Given this risk profile, the absence of standardized dose thresholds against the backdrop of growing CT utilization presents patient-safety and public-health concerns.
In response, CMS contracted the University of California San Francisco in 2019 to develop a formal quality measure addressing radiation dose in diagnostic CT exams.5 Recognizing that dose reduction efforts must preserve diagnostic value, the resulting measure incorporates image noise thresholds and combines three key elements: scanner-reported dose adjusted for patient size, a global noise metric, and classification of each study into one of 18 CT categories defined by body region and clinical indication. Thus, for each category, CMS specifies paired dose-and-noise thresholds and studies whose measured dose or noise fall outside these limits are defined as out of range.5
The benchmarking data underpinning the measure were derived from a registry of 4.5 million adult CT exams, across 383 scanners representing 74 distinct models, in the UCSF International CT Dose Registry. Median and 75th-percentile dose levels varied by up to an order of magnitude within categories, validating the need for category-specific benchmarks and optimization.6 Thresholds were then established based upon image-quality evaluation of 200 CT exams by 125 radiologists such that dose thresholds corresponded to at least 90% of radiologists judging image quality to be adequate, and noise thresholds were defined as the point at which 25% of radiologists rated the image quality as inadequate.7
Following multisite pilot testing, the National Quality Forum endorsed the measure for hospital and physician‐level quality assessment, marking it as the first radiology measure to receive such recognition.5
OK, So We Have a Radiation and Quality Measure. Now What?
Implementing the measure in a practical manner involves conversion to an electronic clinical quality measure (eCQM); it is this eCQM that will be reported to CMS. Because the eCQM framework was not designed to integrate DICOM Radiation Dose Structured Reports or raw image noise data directly, hospitals must translate radiology data into the standardized format required for eCQM submission. However, there is considerable confusion in the general radiology ecosystem, among practices and the marketplace, as to what is actually required in this process by CMS.
To begin with, satisfactory measure reporting can be achieved either through EHR-embedded eCQM modules (such as EPIC) or via third-party software that extracts, normalizes, and submits the data directly to CMS. There is no requirement by CMS to use any particular software vendor. In fact, a clarification to this effect was recently published by CMS on January 30, 2025. Indeed, the market already contains vendors who offer reporting via multiple methods. For example, ALARA and Imalogix both offer FHIR, HL7, and CSV outputs for EHR integration.
Furthermore, as clarified by CMS in the same January 30, 2025 release, no single vendor or proprietary platform is required— any software meeting the measure’s specifications may be used , and hospitals are not obligated to demonstrate vendor approval to CMS.8 In fact, CMS does not—and has never—required that different software from different vendors even produces the exact same output.9, 10 Rather, CMS only requires a software solution that works within the “measure specifications,” i.e., use of the 18 CT categories and use of size-adjusted dose.

While this last point may seem a bit counter-intuitive, it is actually quite consistent with the nature of the measure. To understand how slight variations can arise, it is instructive to look at a specific component. For example, the size-adjusted radiation dose is calculated as
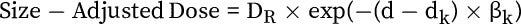
where d is the patient’s effective anatomic diameter—but there are many potential variations in diameter measurement techniques: Is the patient’s diameter measured at one point (ie, the mid-point of the scan) or does it represent an aggregate value (ie, average or median across the scan)? Is diameter measured in the lateral and AP directions to calculate the effective diameter, or is the area of the patient used to compute the effective diameter? d k is the expected, or reference, diameter for the assigned CT category, derived from the registry’s median exam diameters. However, its utilization is not formalized, and other registries and databases may yield small discrepancies in this variable.
Thus, subtle differences in dose calculations can exist across sites and software,8 while still being acceptable to CMS.
The Upshot
Individual practices should survey the landscape and see which solution works best for them, including providers such as Imalogix, ALARA, or others.
The reporting deadlines are approaching, and there are a few avenues of thought and action that should be common to all radiology departments at this time:
-
Validate data pipelines to ensure accurate extraction and mapping of dose and noise metrics to whichever software solution is chosen.
-
Evaluate eCQM solutions for seamless integration with existing EHR and/or PACS workflows.
-
Engage multidisciplinary stakeholders —IT, quality, compliance, and clinical teams—to establish governance for data review and exception management.
-
Monitor performance continuously to identify outliers and drive iterative optimization.
By embracing this standardized measurement framework, institutions can reduce unnecessary radiation exposure, uphold diagnostic quality, and align with CMS’s evolving quality‐reporting landscape, regardless of the specific software solution employed.
References
Citation
Goldberg A.What Does CMS Dose Compliance in Radiology Mean for You?. Appl Radiol. 2025; (3):1 - 3.
doi:10.37549/AR-D-25-0088
June 1, 2025