Vesicouterine Fistula
Images
CASE SUMMARY
A 28-year-old female gravida 5 with 2 living children delivered at term, was admitted at 39 weeks and 2 days of gestation for an elective cesarean section (C-section) because of her history of two previous C-sections. The patient underwent a standard C-section with a Pfannenstiel incision. A Foley catheter was routinely placed intraoperatively and left in place following the surgery. The patient remained in the hospital for typical continued postoperative evaluation. Immediately following her surgery, the patient continued to have gross hematuria and passed a small blood clot into the tubing of the Foley catheter. Her postoperative laboratory workup was also positive for a mild anemia with a hemoglobin level of 9.6 g/dL (decreased from 11.0 g/dL) and a hematocrit level of 29.9% (decreased from 33.0%). The patient was returned to the operating room for a cystoscopy with clot evacuation. No obvious bladder injury was found. The patient remained on continuous bladder irrigation for approximately 24 hours, at which time it was discontinued as her urine had been running light pink to clear. At this time, a CT cystogram was performed.
IMAGING FINDINGS
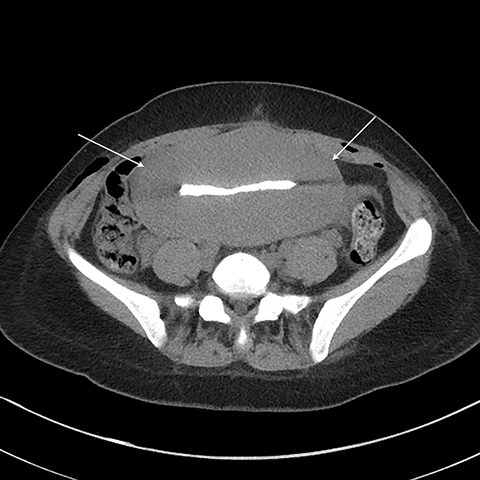
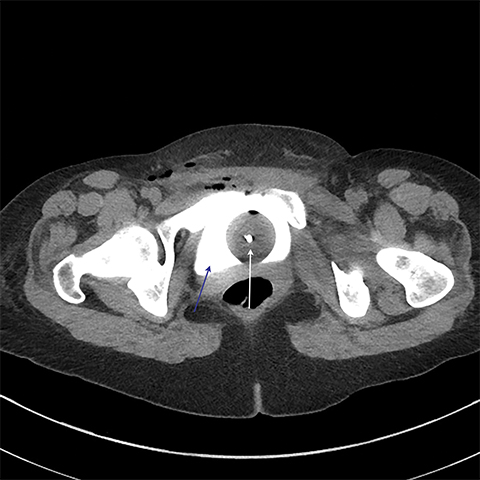
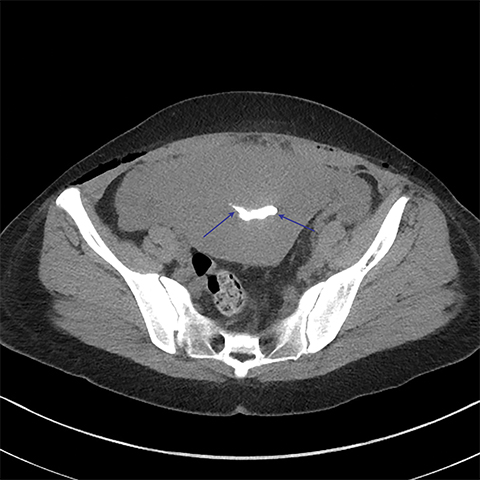
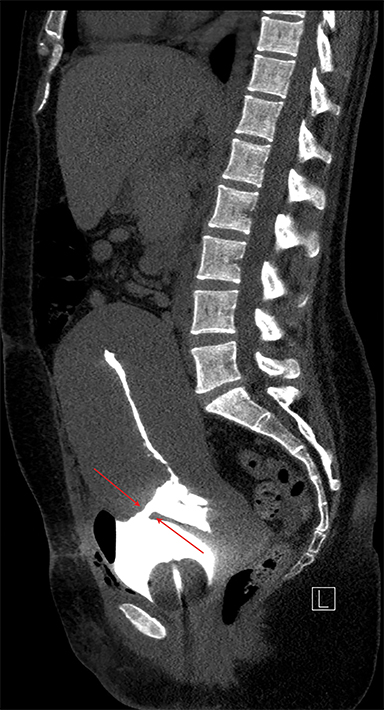
Multiple axial images were obtained through the pelvis in 5 mm slices following instillation of 400cc of Cysto-Conray through the patient’s existing Foley catheter as per our standard CT cystogram protocol. Post-gravid appearance of the uterus and postoperative changes related to a recent cesarean section were demonstrated (Figure 1). A Foley catheter balloon was noted to lie within the lumen of the bladder (Figure 2). Contrast was noted to extend from the lumen of the bladder into the endometrial cavity at the level of the prior incision along the lower uterine segment through a small fistula (Figure 3). A conservative approach to treatment of the fistula with continued decompression of the urinary bladder was undertaken with a Foley catheter for 1 week. Follow-up cystograms were obtained at 1 week and 4 months following surgery. There was no evidence of the previously demonstrated vesicouterine fistula on either follow-up cystogram.
DISCUSSION
Urogenital fistulas are classified according to their anatomic location; they include vesicovaginal, vesicocolonic, vesicoperitoneal, ureterovaginal, and vesicouterine fistulas. The overall incidence of urogenital fistulas in the United States is reported to be between less than 0.5% and 10% after surgical intervention.1 Vesicouterine fistulas are the least common form, accounting for 1-4% of reported cases.1,2
Most vesicouterine fistulas in the United States occur after cesarean section, but they may also be secondary to intrauterine contraceptive devices, tumors, or traumatic labor. Other far less frequent etiologies include radiation therapy or iatrogenic causes, such as traumatic catheterizations.3,4 The reported incidence of urogenital fistulas is thought to be increasing secondary to an increasing rate of cesarean deliveries. From 1996 to 2009, the rate of cesarean deliveries increased from 20.7% to 32.9%. The cesarean delivery rate for 2015 slightly decreased and was estimated to be 32%; however, the data did not take into account the declining birth rate in the U.S. Overall, the percentage of cesarean deliveries, as well as the total number of births, have since continued to trend upward.5,6
Delays in the diagnosis of urogenital fistulas are not uncommon, due to delays in the onset of symptoms and, at times, a nonspecific clinical presentation. Imaging studies such as cystography, sonography, computed tomography (CT), or magnetic resonance (MR) can aid in the diagnosis of urogenital fistulas.7
The diagnosis can be made following a thorough history and physical examination, which may demonstrate urine pooling within the vaginal vault. Diagnosis can also be made with cystography, cystoscopy, dye testing, intravenous pyelography (IVP), and/or cross-sectional imaging.7 However, the findings of such studies are commonly inconclusive, depending on the size and characteristics of the fistulous tract. False-negative IVP’s are common, as these studies may not generate adequate intraluminal pressure within the bladder to opacify the fistula.8 For smaller fistulas, a dye test or cystography is the initial imaging test of choice. If the findings are inconclusive or clinical suspicion of a fistula remains high, additional evaluation with cross-sectional imaging with intraluminal contrast, including coronal and sagittal reformats, is generally warranted.7-9
If a vesicouterine fistula is not recognized and repaired at initial surgery, continuous urinary drainage can sometimes be effective. Additional therapeutic options also include fibrin-based surgical sealants or resection of the fistulous tract utilizing a laparoscopic/robotic/vaginal approach. Of note, the rate of spontaneous healing of the vesicouterine fistula in patients solely undergoing continuous bladder decompression has been reported at 5%.10-16
CONCLUSION
The clinical presentation of our patient was atypical in the sense that it was such a short time interval from C-section to symptom presentation, which primarily consisted of frank hematuria. In general, patients with a vesicouterine fistula typically present with urine leakage through the vagina, which tends to become more noticeable a few days post-op following removal of the Foley catheter.
Given the small size of the fistulous tract in this case, the patient elected to undergo conservative therapy, with successful healing of the fistula on subsequent imaging studies.
REFERENCES
- Mann WJ, Arato M, Patsner B, Stone ML. Ureteral injuries in an obstetrics and gynecology training program: etiology and management. Obstet Gynecol. 1988; 72:82.
- Dodero D, Corticelli A, Caporale E, Cardamone C. Endometriosis arises from implant of endometriotic cells outside the uterus: a report of active vesicouterine centrifugal fistula. Clin Exp Obstet Gynecol. 2001; 28:97-99
- Tancer ML. Vesicouterine fistula: a review. Obstet Gynecol Surv. 1986; 41:743-753
- Hodonou R, Hounnasso PP, Biaou O, Akpo C. Vesicouterine fistula: report on 15 cases at Cotonou University Urology Clinic. Prog Urol. 2002; 12:641-645
- Osterman MJK, Martin JA. Trends in low-risk cesarean delivery in the United States,1990–2013. National vital statistics reports; vol 63 no 6. Hyattsville, MD: National Center for Health Statistics.2014.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2015. NCHS data brief, no 258. Hyattsville, MD: National Center for Health Statistics. 2016.
- Abou-El-Ghar ME, El-Assmy AM, Refaie HF, El-Diasty TA. Radiological diagnosis of vesicouterine fistula: role of magnetic resonance imaging. J Magn Reson Imaging. 2012; 36:438.
- Smayra T, Ghossain MA, Buy JN, et al. AJR. 2005 184:1, 139-142
- Singh O, Gupta SS, Mathur RK. Urogenital fistulas in women: 5-year experience at a single center. Urol J. 2010; 7:35.
- Hadley HR. Vesicovaginal fistula. Curr Urol Rep. 2002; 3:401.
- Garza Cortés R, Clavijo R, Sotelo R. Laparoscopic treatment of genitourinary fistulae. Arch Esp Urol. 2012; 65:659.
- Liao CY, Tasi RS, Ding DC. Gynecological surgery caused vesicovaginal fistula managed by Latzko operation. Taiwan J Obstet Gynecol. 2012; 51:359.
- Demirci U, Fall M, Göthe S, et al. Urovaginal fistula formation after gynaecological and obstetric surgical procedures: clinical experiences in a Scandinavian series. Scand J Urol . 2013; 47:140.
- Hoch WH, Kursh ED, Persky L. Early, aggressive management of intraoperative ureteral injuries. J Urol. 1975; 114:530.
- Boateng AA, Eltahawy EA, Mahdy A. Vaginal repair of ureterovaginal fistula may be suitable for selected cases. Int Urogynecol J. 2013; 24:921.
- Schlossberg SM. Ureteral healing. Semin Urol. 1987; 5:197.
Citation
J E, RE G, A K, P P, D P, K T, J B, A M.Vesicouterine Fistula. Appl Radiol. 2020; (1):56A-56C.
January 23, 2020