Vaccine-Related Lymphadenopathy Lasts Longer Than Previously Reported
 COVID-19 mRNA vaccine-related axillary lymphadenopathy detected by breast ultrasound lasts longer than reported in initial vaccine clinical trials, according to a study published in the American Journal of Roentgenology (AJR).
COVID-19 mRNA vaccine-related axillary lymphadenopathy detected by breast ultrasound lasts longer than reported in initial vaccine clinical trials, according to a study published in the American Journal of Roentgenology (AJR).
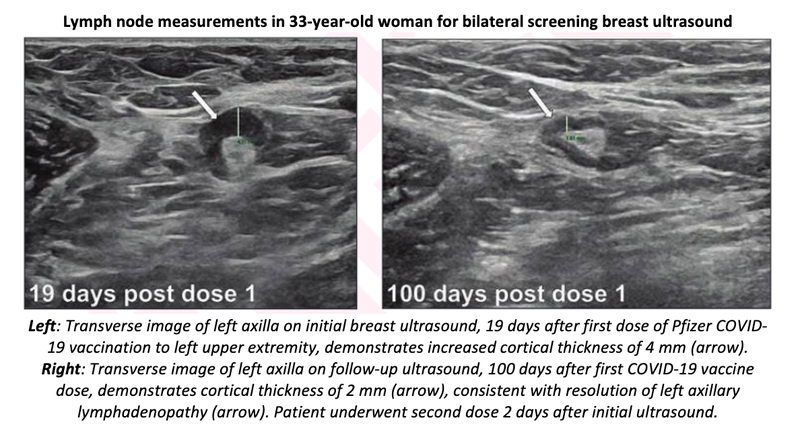
“The prolonged resolution time supports a follow-up interval of at least 12 weeks for suspected vaccine-related lymphadenopathy and avoidance of delaying screening mammography after vaccination,” wrote corresponding author Michele B. Drotman, MD.
Dr Drotman and the Weill Cornell Medicine team extracted health record data for 111 patients (mean age, 52 years) with unilateral axillary lymphadenopathy ipsilateral to administration of Pfizer or Moderna COVID-19 vaccine—performed within 8 weeks prior and detected on breast ultrasound (January 1–October 1, 2021) that underwent follow-up ultrasound examinations at 4–12 weeks.
In this single-center study, axillary lymphadenopathy ipsilateral to COVID-19 mRNA vaccination resolved after a mean of 97 days since detection by breast imaging and 127 days since the first dose. Longer times to resolution were observed with Moderna (rather than Pfizer) vaccination, receipt of second dose after presentation, and thicker cortical thickness at presentation.
“The presence of subclinical lymphadenopathy and the long resolution time of lymphadenopathy,” the authors of this AJR article noted, “should reassure radiologists and patients when lymph nodes suspected to be vaccine-related persist over multiple visits.”