US FDA Clears SpinTech MRI Quantitative Brain Imaging Technology
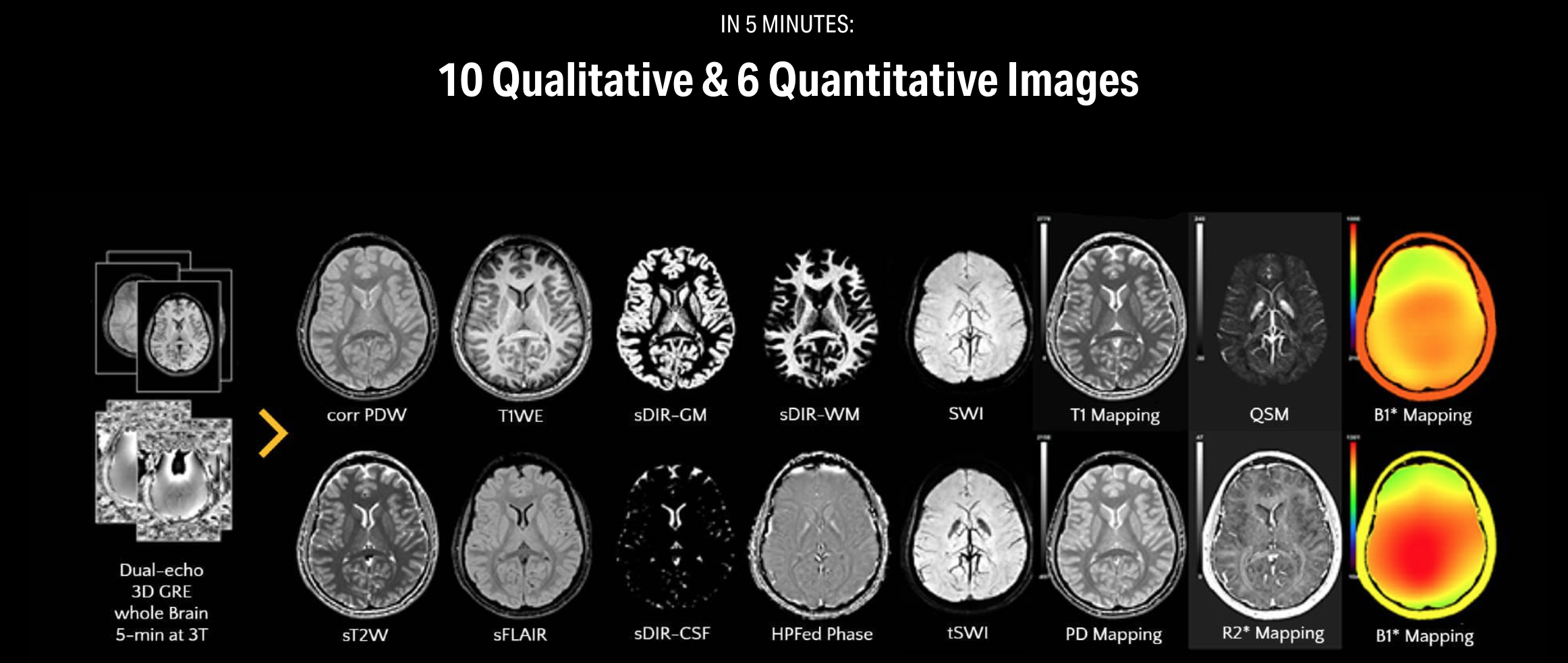 SpinTech MRI’s latest magnetic resonance imaging software device, STAGE (STrategically Acquired Gradient Echo), has received US Food and Drug Administration 510(k) clearance. STAGE is a post-processing software platform that enables comprehensive, quantitative brain imaging with enhanced visualization in significantly less time than conventional approaches. It allows MRI technicians to acquire 16 brain imaging contrasts, including 10 enhanced contrast qualitative outputs and 6 quantitative outputs in just 5 minutes on 3T systems. STAGE is cleared for use on both 1.5T and 3T systems across manufacturers, and runs on existing equipment.
SpinTech MRI’s latest magnetic resonance imaging software device, STAGE (STrategically Acquired Gradient Echo), has received US Food and Drug Administration 510(k) clearance. STAGE is a post-processing software platform that enables comprehensive, quantitative brain imaging with enhanced visualization in significantly less time than conventional approaches. It allows MRI technicians to acquire 16 brain imaging contrasts, including 10 enhanced contrast qualitative outputs and 6 quantitative outputs in just 5 minutes on 3T systems. STAGE is cleared for use on both 1.5T and 3T systems across manufacturers, and runs on existing equipment.
“STAGE’s FDA clearance is a huge breakthrough for SpinTech MRI,” said Ward Detwiler, SpinTech MRI’s CEO. “After years of developing and refining the platform through extensive research use, we are incredibly excited to make this game-changing technology available for clinical use in hospitals and imaging centers.”
STAGE also generates quantitative outputs such as T1 and proton density (PD) maps. According to SpinTech MRI, it is the first 510(k) cleared product to provide Quantitative Susceptibility Mapping (QSM). Through the company’s extensive research partnerships, they have established powerful clinical use cases across numerous diagnostic areas. When interpreted by a trained physician, STAGE-processed images may provide information useful in determining diagnosis for stroke, dementia, Parkinson’s, multiple sclerosis, tumors and more.
“Radiologists have long struggled to obtain comprehensive, high-quality clinical data within very constricted scanning windows,” said Dr. Mark Haacke, PhD, Founder and Chief Science Officer at SpinTech MRI. “Now, they don’t need to choose which sequences to run, just what they are going to examine, as all the data they need has already been collected. On top of the enhanced imaging data, radiology groups will also benefit from increased patient throughput. And, of course, patients experience shorter scan times, so everybody wins.”
Hospitals or imaging centers can improve the efficacy of their MR imaging systems, both old and new, without taking machines offline or investing in expensive hardware. STAGE is compatible with nearly any existing MRI, providing standardized image outputs across different manufacturers and enabling more reliable longitudinal comparison across different machines.
“We have seen, pretty consistently, that reimbursement rates are declining while demand for advanced MRI has increased, putting a lot of pressure on imaging groups,” said Detwiler. “Technology solutions that improve both cost and workflow efficiency of imaging procedures are paramount to hospitals and imaging centers, and STAGE perfectly fits that bill.”
Though STAGE is considered a comprehensive brain imaging post-processing solution, its approval does not extend outside of brain uses and should always be used in combination with at least one other conventional MR acquisition technique. The software is currently in use in over 50 hospitals, imaging centers and research facilities around the globe.
Related Articles
Citation
US FDA Clears SpinTech MRI Quantitative Brain Imaging Technology. Appl Radiol.
August 10, 2021