Technology Trends—RSNA 2014: A celebration of the past and future
Images






Throughout the 2014 annual meeting of the Radiological Society of North America (RSNA), it was hard to escape the fact that this was a milestone celebration.
From the theme of the President’s Address to the Centennial Showcase, which featured a century of radiology innovation, to the life-sized model of Wilhelm Roentgen and even artwork created from analog X-rays, RSNA 2014 was a meeting of reflection on past achievement and reinvigoration for future endeavors.
In his opening Plenary Session, RSNA President N. Reed Dunnick, MD, called upon radiologists and the organization to continue adapting to the future of medicine. Besides calling for continued research and education, Dr. Dunnick also reiterated the need for radiology to continue partnering with other specialties and imaging equipment manufacturers. The key to radiology’s future, he said, is the ability to bring technological and scientific advancements together with patient-centered care. He said radiologists can become more relevant and valuable by connecting with patients and other members of the healthcare team, making the profession itself stronger in the process. Value—not volume—is the new paradigm, Dr. Dunnick added, noting how advances in ultrasound, CT, and MRI have helped to transform medicine.
Francis S. Collins, MD, PhD, Director of the National Institutes of Health (NIH), presented the opening session’s Special Lecture, discussing how imaging-based research helps propel major breakthroughs in healthcare. He called for reinvigorating biomedical research, translating science into treatment, and initiating greater collaboration among members of the RSNA and other radiological stakeholders.
In terms of attendance, there’s nothing like a 100-year celebration to attract crowds. While exhibitor attendance was down about 2% from 2013, guest attendance was up an average of almost 21% across all 6 days of the meeting, with a total overall attendance increase of 5.5% over 2013’s annual meeting attendance. The takeaways for all these attendees fell into several clear-cut trends, including—somewhat surprisingly—the growing popularity of ultrasound imaging.
The year of ultrasound?
After many years in which “big ticket” modalities—think CT, MRI, and PET—have garnered most of the spotlight at RSNA, ultrasound seemed to grab much of the attention at 2014’s meeting. Some of the excitement, no doubt, was partially ignited by the modality’s value in imaging dense breasts—an especially hot topic in sessions and on the showroom floor. In addition to 19 states with legislation on the books requiring women be notified of their breast density, national legislation imposing similar requirements is being considered. Along with MRI, ultrasound is a viable screening alternative for this patient population.
In his New Horizons Lecture, Jonathan M. Rubin, MD, PhD, director of the Division of Ultrasound at the University of Michigan, spoke of volume blood flow and elastography utilizing shear wave imaging.
Philips Healthcare, meanwhile, announced the formation of a consortium of healthcare leaders to help drive mobile ultrasound solutions. While the company is still actively seeking clinicians for this multi-disciplinary group, the company announced the inaugural members, including Jagat Narula, MD, of Mount Sinai Hospital; Norman J. Beauchamp, MD, of The University of Washington; and Michael B. Stone, MD, FACEP, of Brigham and Women’s Hospital. Philips said the consortium will help develop mobile, app-based ultrasound solutions to interface with the Philips HealthSuite Digital Platform, the goal being to extend ultrasound’s reach into the hands of more healthcare professionals.
In the exhibit halls, meanwhile, GE Healthcare showcased its LOGIQ E9 ultrasound system, whose elastography capability measures the speed of shear waves as they travel through tissue and displays results in 2D, real-time, color-coded ultrasound: low speed corresponds to softer tissue and high speed corresponds to stiffer tissue (Figure 1).
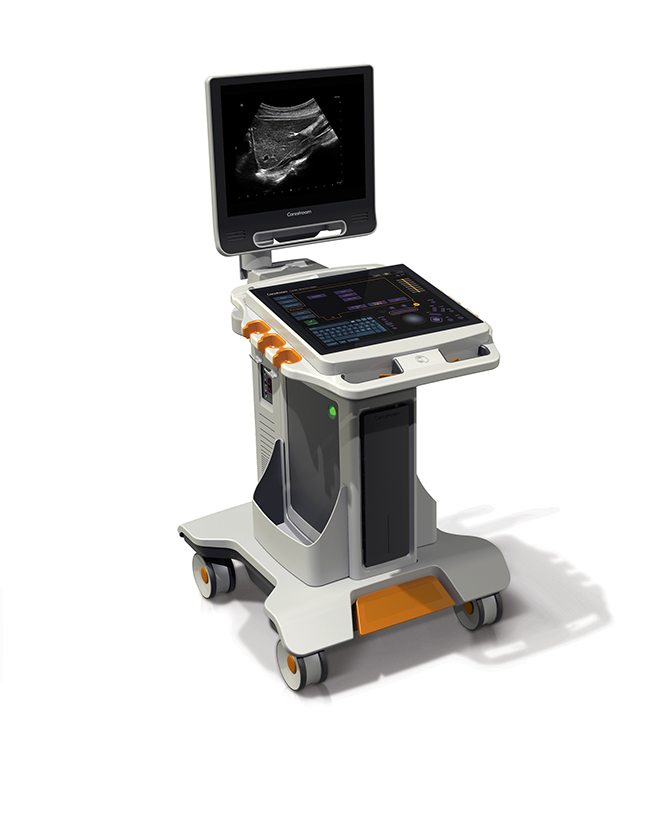
Carestream announced its entry into ultrasound with its first system, the Touch Ultrasound System. The all-touch control panel, integrated GPU processing power, and smart transducer technology, coupled with a single-board system design, provides a compact footprint and modern user interface. Etched marking for primary controls enables users to locate key functions without looking away from the image display monitor (Figure 2).
Konica Minolta Medical Imaging launched its second ultrasound system—the new SONIMAGE HS1, a hand-carried system that features best-in-class imaging technology with advanced transducer and tissue harmonic imaging technology. The SONIMAGE HS1’s broadband linear probe features several advancements in transducer technology, such as a multilevel matching layer, optimized component materials, and nanofabrication technology. The L18-4 probe can be used to image both the tiny details of fingers and the larger features of areas such as the hips and shoulders. The probe also provides simple needle visualization—the tip appears blue in relation to surrounding tissue—and does not rely on beam steering (Figure 3).
FUJIFILM SonoSite unveiled its X-Porte Ultrasound Kiosk for point-of-care, portable ultrasound imaging. The foundation of X-Porte is Sono-Site’s proprietary Extreme Definition Imaging™ (XDI) technology, a new beam-forming algorithm that significantly reduces visual clutter from side-lobes that affect all ultrasound products regardless of system size. The resulting ultrasound image appears cleaner with optimal tissue differentiation (Figure 4).
Siemens Healthcare, meanwhile, showcased its X-class of mid-range ultrasound systems, which are geared toward reducing variability and enhancing image quality and now include the ACUSON X600 and the ACUSON X700. A key feature of the ACUSON X600 is the Dynamic TCE tissue contrast enhancement technology that helps enhance border detection and reduce image artifacts. The 2.0 release of the ACUSON X700 includes eSie Touch elasticity imaging for noninvasive relative tissue stiffness analysis (Figure 5).
Toshiba America Medical Systems announced that its new Aplio 300 and 500 Platinum Series will help healthcare providers and facilities meet the CMS’ goals for Triple Aim, which is focused on improving the patient experience of care, improving the health of populations, and reducing the per capita cost of healthcare. Key features of the Aplio Platinum series designed to fill these goals include BEAM, a new technique for improved needle visualization; shear wave elastography for noninvasive liver stiffness measurements; and Superb Micro-Vascular Imaging (SMI) to capture low-velocity blood flow without contrast agents.
SuperSonic Imaging received FDA clearance for new musculoskeletal and vascular ultrasound probes for its Aixplorer. The SHL20-6 probe is a high-frequency transducer shaped like a hockey stick that facilitates musculoskeletal and sports medicine diagnoses. It provides high-resolution images of muscles, tendons, ligaments and joints and complements the linear transducer range to provide high image quality for both very superficial and deep zones. The company’s ShearWave Elastography system, which runs on the Aixplorer, allows practitioners to view and measure tissue stiffness of up to 800kPa in real-time. The second probe is the single crystal XP5-1 transducer, which combines high sensitivity and enhanced resolution to visualize very low blood flow—an important feature for optimal transcranial Doppler (TCD) and abdominal vascular measurements.
Other notable ultrasound product introductions at RSNA included the RS80A, a high-resolution, full-featured, premium ultrasound system from Samsung Electronics Co., Ltd.; Analogic Corporation’s bk3000, which the company is targeting to interventional and image-guided procedures, thanks to the TriCore architecture that provides ultra-high resolution and Doppler imaging capabilities; and a new 3D ultrasound, GOPiCE 2.0, from ContextVision.
Computed tomography
Thanks in part to a Nov.10, 2014 proposed memo from the Centers for Medicare & Medicaid Services (CMS) recommending insurance coverage of low-dose CT lung cancer screening tests in certain senior populations, computed tomography also garnered its share of attention at RSNA 2014. Indeed, a number of CT manufacturers indicated they either had filed or were in the process of filing with the FDA for 510(k) clearance of their systems for lung cancer screening.
For its part, Philips Healthcare used the meeting to launch its own integrated patient management solution for lung cancer screening. Billed as a “total solution comprised of products and services,” the solution includes radiology workflow, services marketing, patient management, and physician education resources. The company says it can work with most low-dose CT scanners (Figure 6).
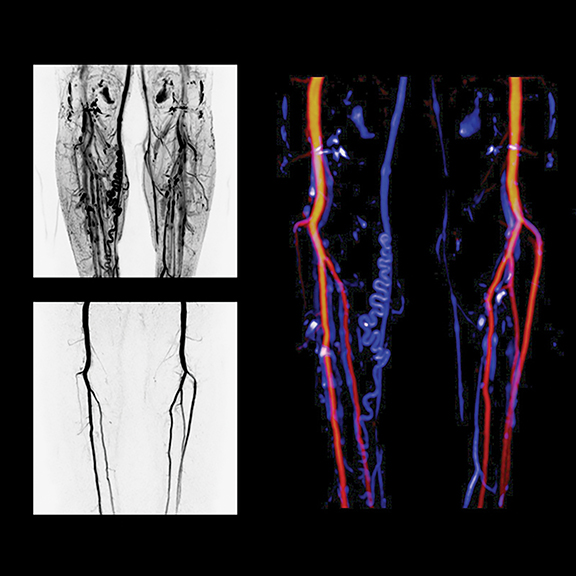
Low-dose CT, buoyed by lung cancer screening developments, and dual-energy imaging were key highlights in most CT exhibits. Siemens Healthcare introduced the SOMATOM Definition Edge CT system for acquiring single-source dual-energy images. The TwinBeam Dual Energy X-ray tube concept of the relaunched SOMATOM Definition Edge enables simultaneous imaging at two different energy levels for the first time in single-source computed tomography (CT), according to Siemens, which also introduced iMAR, a new iterative algorithm to reduce metal artifacts. Currently installed SOMATOM Definition Edge and SOMATOM Definition AS+ CT scanners can be retrofitted with TwinBeam technology, iMAR and ADMIRE (in combination with the Stellar detector). iMAR, TwinBeam and SOMATOM Definition Edge are all pending US FDA 510(k) clearance (Figure 7).
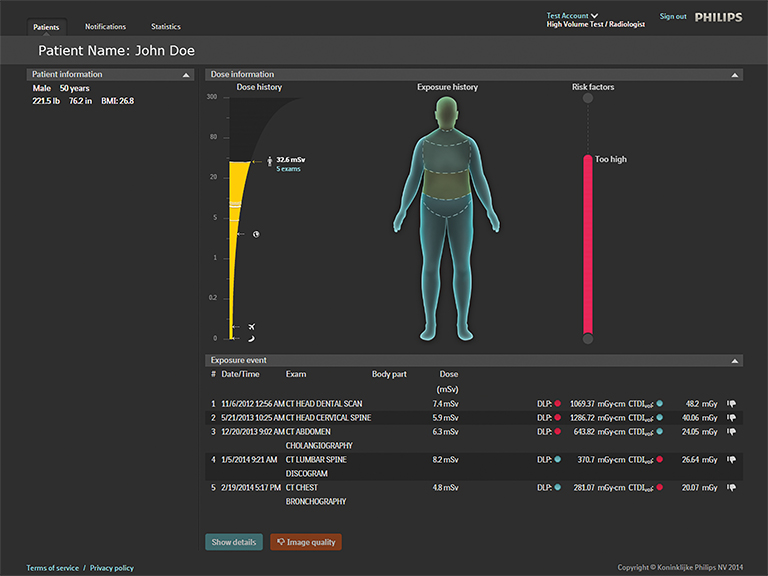
Philips also launched its DoseWise Portal at RSNA 2014. This new integrated radiation dose management solution will enable healthcare providers to proactively record, analyze and monitor imaging radiation dose for patients and clinicians across multiple diagnostic settings. It includes a comprehensive portfolio of products and services from the company, including ClarityIQ, IMR and DoseAware (Figure 8).
Unfors RaySafe also announced that it has inked an exclusive agreement with Philips to license the cloud-based patient dose tracking system, Patient Dose Management System RaySafe S1. As a result of the agreement, Unfors RaySafe will no longer market or sell its product, and the technology will become part of Philips’ DoseAware family of products.
CT radiation dose was also a key focus at GE Healthcare, which launched ASiR-V for dose management and the Revolution EVO CT system, which the company says will enable clinicians to scan more patients with high image quality at a low dose. ASiR-V incorporates modeling characteristics of Veo to enable dose reductions of up to 82% on the Revolution EVO, which also delivers 20%-higher spatial resolution and 25-40%-lower noise than previous GE systems (Figure 9).
Works-in-progress enhancements to Toshiba’s flagship Aquilion ONE CT were focused on lowering dose and enhancing patient satisfaction. These include AIDR 3D Enhanced, an iterative noise reduction algorithm for improving image quality in low-dose CT acquisitions; thePUREVISION CT Detector that the company says results in 40% better light output for more efficient use of X-ray photons; andSUREKV for automated kV selection based on patient size (Figure 10).
MedicVision showcased its enterprise edition of Safe CT and highlighted the latest findings on dose reduction using the Safe CT solution at Massachusetts General Hospital in a series of four presentations. Atul Padole, MD, shared data demonstrating that Safe CT, which can be installed on existing CT systems, delivers comparable results for dose reduction and image quality as other industry-leading dose management solutions. Dr. Padole said his study demonstrates that hospitals don’t need to replace their CT system just to lower dose and that other options are available.
Bracco Diagnostics, Inc., introduced the ISOVUE Imaging Bulk Package* (IBP). According to Bracco, it is the only specific combination multi-patient, multi-dose-compliant contrast medium approved by the US FDA for point-of-care use in the CT suite. The ISOVUE IBP was designed to increase safety and improve workflow, while minimizing risk and maintaining compliance with FDA rules on the use of multi-dose contrast media for radiological procedures in the CT suite. The ISOVUE IBP provides 10-hour, on-demand dosing from the same container without the risk of incurring multi-dosing citations associated with transferring contrast media outside the hospital pharmacy setting.
Magnetic resonance
Magnetic resonance imaging manufacturers continued to focus on “value MR” at RSNA 2014, bringing advanced imaging features found on premium systems to productivity or workflow-based solutions geared more for the community hospital market. In addition, there were two significant MRI news items at this year’s meeting—one related to technology, and one related to contrast media.
New data presented at RSNA reported that the pharmacokinetic profile and safety of Bayer’s Gadavist (gadubutrol) at a standard dose (00.1 mmol/kg) in children under 2 years was similar to adults and children over 2 years. Armed with the study results, Bayer received U.S. FDA approval on Dec.29, 2014 through a priority review expanding the label for this contrast media in pediatric patients under 2, said Denny Durmis, Vice President and Head of the Americas Region, Bayer Radiology. With approval, clinicians will be able to utilize Gadavist with MRI to detect and visualize areas with disrupted blood-brain barrier and/or abnormal vascularity of the central nervous system, Durmis explained. “We’ll continue to invest in other indications for Gadavist in the U.S. market.”
In addition to showcasing the recently FDA-cleared SIGNA PET/MR, the first time-of-flight-capable, integrated, simultaneous PET/MR system, GE Healthcare also displayed two new FDA-pending 1.5T MR systems: the SIGNA Creator and the SIGNA Explorer. Both are designed for lower total cost of ownership by using 34% less power than previous generations. GE also showed off some MAGiC (Magnetic Resonance Image Compilation)—its FDA-pending technique that gives clinicians the ability to generate multiple image contrasts in a single MRI scan of the brain, including T1-, T2-, STIR, T1 FLAIR-, T2 FLAIR- and PD-weighted imaging. According to GE Healthcare, MAGiC allows the acquisition of six contrasts in as little as one-third the time, which could enable the scanning of one more patient per hour, every hour of the day in neuro imaging. The tool is the result of a collaboration with SyntheticMR AB.
At Siemens, in addition to the works-in-progress MAGNETOM Amira, there was a strong MR focus on prostate imaging with the 510(k)-pending SEEit MR application, which enables performance of a noninvasive prostate MR exam without an endorectal coil. With the SEEit application, the company says, clinicians can conduct a 10-minute multiparametric prostate exam—with morphological and diffusion weighted imaging data—by using the new Body 60 channel coil, a high-density, 16-channel surface coil also pending FDA clearance. The MAGNETOM Amira 1.5T system features Eco-Power technology that enables up to 30% power savings in standby mode (Figure 11).
ABSIST, LLC, announced an agreement with Siemens Healthcare for a non-exclusive, worldwide license covering its patented technology for automated spinal detection, labeling and prescriptions. The Automated Spine Survey Iterative Scan Technique (ASSIST) was first presented at RSNA in 2004 and subsequently published in Radiology in 2006.
Philips launched the Ingenia 1.5T S, which features fat-free and motion-free imaging techniques along with the company’s patient in-bore solution where patients can choose a visual theme to fill the room with colorful video images. Designed for the company’s “First Time Right” imaging and aimed at reducing patient anxiety and enabling faster workflow, the system also includes ComforTone scan techniques to reduced scanner noise.
Toshiba launched its 1.5T MR value system—the Vantage Elan—with what the company says is the smallest footprint in its class at 23 square meters and lower total cost of ownership. The system also includes the company’s Pianissimo noise-reduction technology, noncontrast imaging techniques, and Intuitive M-Power user interface for improved productivity, simplified exams and advanced post-processing tools (Figure 12).
Women’s health
Walking the RSNA exhibit halls couldn’t help but bring to the mind of many attendees (this one at least), that this year was also all about the breasts. From the Dangerous Boobs Tour that stopped at RSNA, sponsored by SonoCine AWBUS (Automated Whole Breast Ultrasound), to Hologic proudly pointing out the 71 posters and presentations on 3D mammography at the meeting, there was no escaping the fact that RSNA in many ways was densely focused on the breasts. Most notable among many studies was one comparing cancer detection using full-field digital mammography (FFDM) alone versus FFDM-plus-digital breast tomosynthesis in 25,547 women between the ages of 50 and 69. FFDM-plus-tomosynthesis pinpointed 80% of the 132 cancer cases in women with dense breasts, compared to only 59% for FFDM alone.
David A. Strahle, MD, President of Regional Medical Imaging (Flint, MI), shared the results of his study and experience using MRI for screening women with dense breasts. Dr. Strahle’s rapid screening breast MRI protocol uses 4 MR acquisitions and requires 7.5 minutes of scan time for a procedure cost of just under $400, or only $150 more than a mammogram.
In a sign of how important patients and consumers have become in the fight against breast cancer, Hologic showcased its “Genius 3D Mammography” consumer awareness campaign launched in October in conjunction with Breast Cancer Awareness Month. Hologic also highlighted its contrast-enhanced mammography application—with one injection clinicians can obtain morphological and uptake/functional imaging data on both breasts.
“Different tests for different breasts” was the theme for GE Healthcare’s Women’s Health segment. Having just received FDA clearance of SenoClaire in early September, GE highlighted its contrast-enhanced spectral mammography, molecular breast imaging and breast MRI in addition to 3D breast tomosynthesis. The company also highlighted its Invenia ABUS solution (Figure 13) and a recently published study in Radiology that concluded screening densely breasted women with ABUS improved breast cancer detection by an average of 27% over mammography alone.1
Volpara launched what it says is the first breast density assessment tool available for clinical use with digital breast tomosynthesis datasets from multiple manufacturers. VolparaDensity 3.0 for Hologic tomosynthesis systems will be available immediately and the new version for GE tomosynthesis units will be available in early 2015.
As 3D tomosynthesis has continued to gain widespread acceptance, VuComp is addressing the need for a CAD solution specifically designed to analyze the vast amount of information that tomosynthesis provides. The company also announced enhancements to MVu CAD version 3.0 and 3.1 that the company says will provide radiologists with a substantial reduction in false positive marks and a lower false positive rate while improving sensitivity.
FUJIFILM enhanced its digital mammography solution—Aspire Cristalle—with Comfort Paddles to enhance the patient experience, the Hexagonal Close Pattern (HCP) detector pixel design for finer detail, higher DQE and lower dose, and the company’s iAEC and ISC image processing technology (Figure 14).
On the MAMMOMAT Inspiration Prime Edition digital mammography system, Siemens replaced the scatter radiation grid common to conventional mammography with Siemens’ Prime algorithm for progressive image reconstruction. According to Siemens, this helps lower patient dose by up to 30% compared to the prior model.
MammoCoach addressed the need for continuing education and quality control in mammography with its internet-based screening mammography training site that simulates real-life scenarios and results. The site includes a chatroom for peer review by individuals or institutions. Participants who complete the program are eligible for 35 hours of Category 1 CME credits.
RSNA 2014 also featured a host of technological and clinical news and developments in molecular imaging, interventional imaging, digital radiography, enterprise imaging and radiology information technology. Applied Radiology will publish coverage of these topics in the February 2015 issue.
Reference
- Brem RF, Tabar L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SonoInsight Study. Radiology. Oct 2014.
Citation
Technology Trends—RSNA 2014: A celebration of the past and future. Appl Radiol.
January 23, 2015