Sylvian Fissure Lipoma with Associated Focal Cortical Dysplasia and Venous Malformation
Affiliations
- 1 Department of Radiology, Catholic Hospital Our Lady of Good Council, Tirana, Albania
- 2 Department of Neuroradiology, Ca’ Foncello Hospital, Treviso, Italy
- 3 Department of Neuroradiology, Mother Teresa University Hospital Center, Tirana, Albania
Case Summary
An asymptomatic adolescent who developed a scalp hematoma following a fall underwent a head CT to rule out parenchymal injury. When an incidental finding was noted, the patient was referred for further evaluation with an MRI.
Imaging Findings
Head CT ( Figure 1 ) revealed a fat-attenuation lobulated mass overlying the right Sylvian fissure, surrounded by abnormally thick underlying cortex. Brain MRI ( Figure 2 ) demonstrated a well-defined lobulated extra-axial right Sylvian fissure mass with high signal intensity in T1 and T2, surrounded by chemical shift artifact. The lesion was encased by a gray matter-lined cortical cleft. The signal dropped out in fat saturation sequences. No enhancement was noted except for the venous component traversing through the lesion.
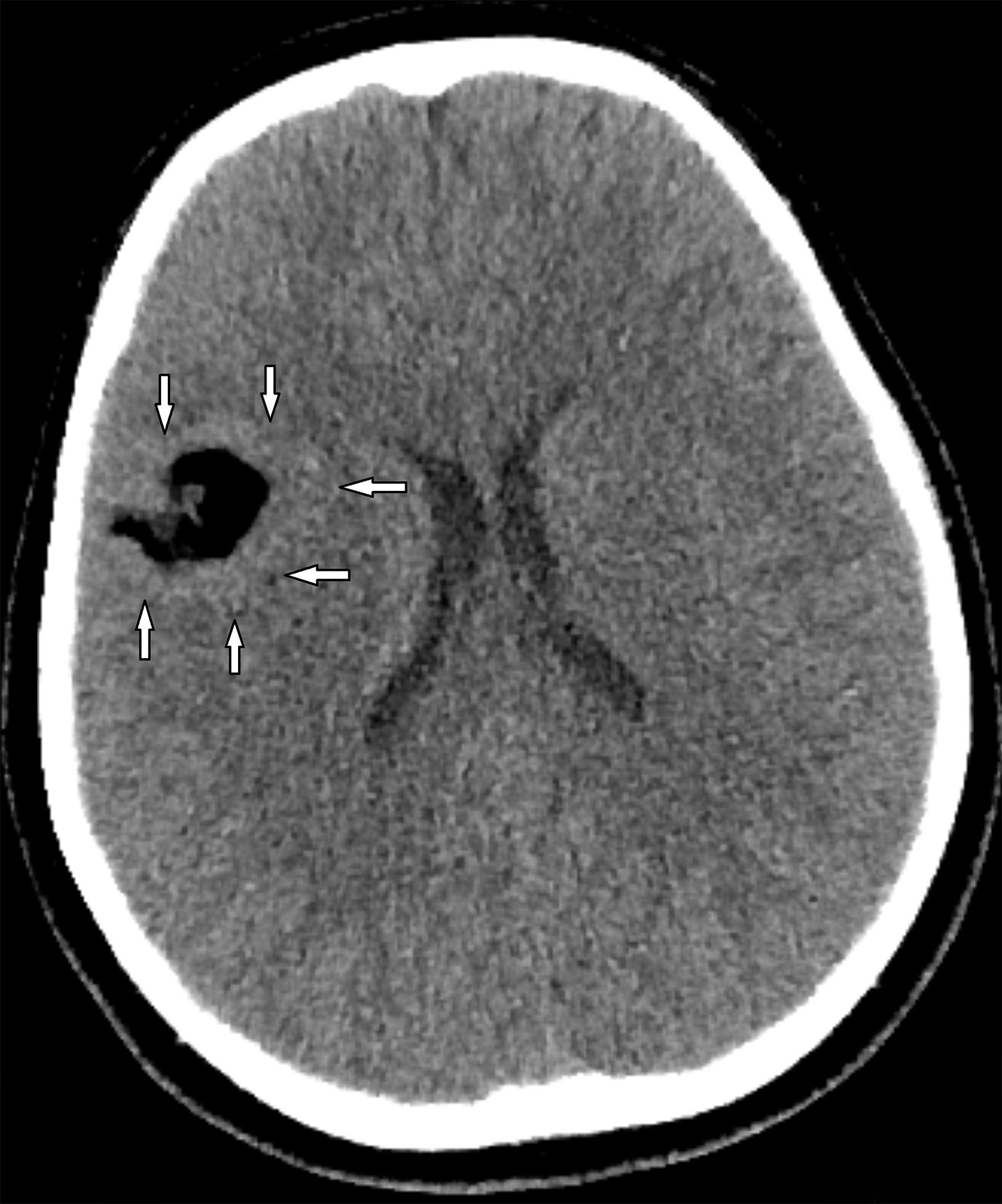
Axial CT image demonstrates a well-defined extra-axial mass of fat attenuation with lobulated margins centered in the right Sylvian fissure. The surrounding cortex appears abnormally thickened (arrows).
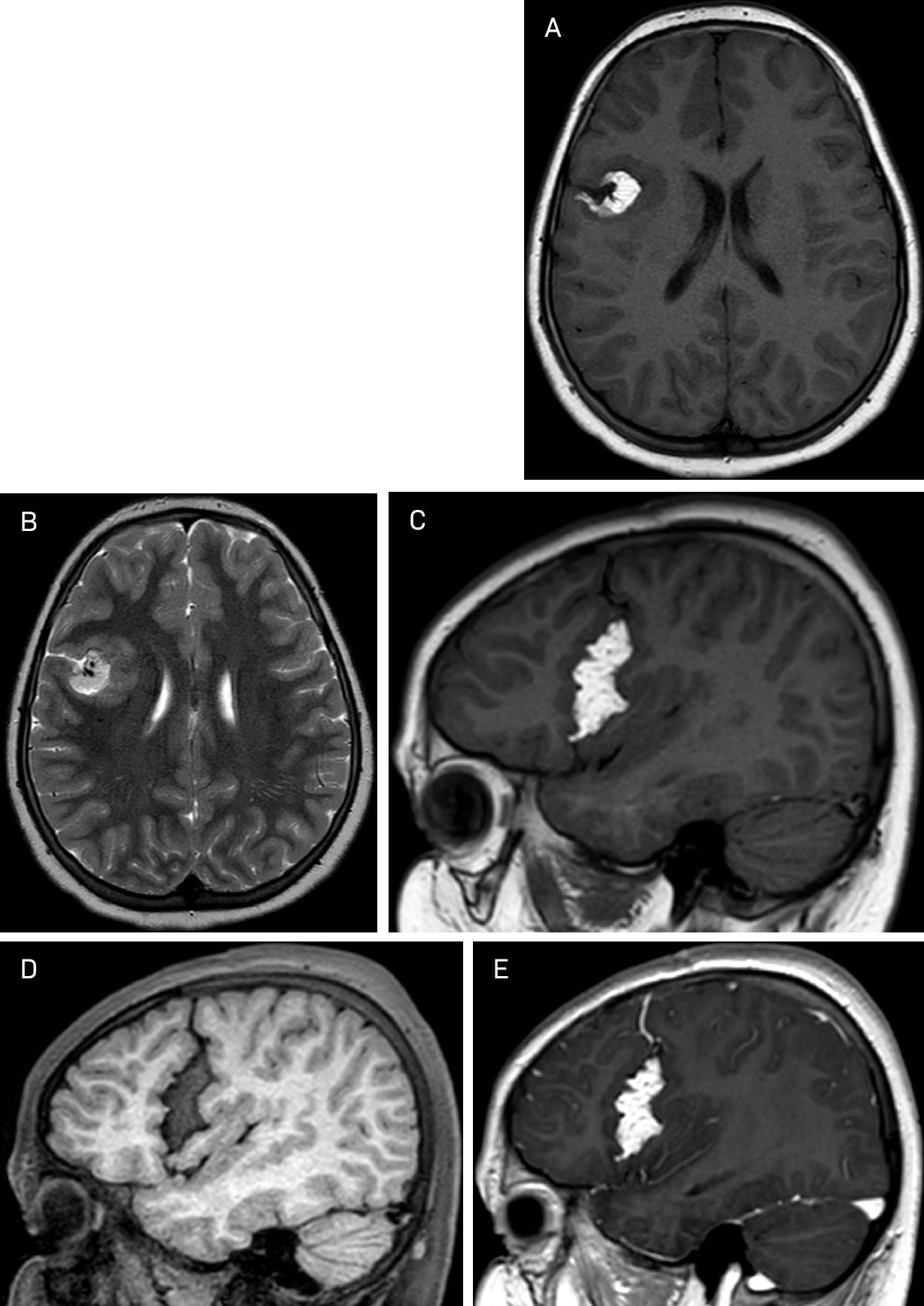
(A, B, C) Axial T1, T2, and sagittal T1 show a well-defined high signal intensity extra-axial mass in the right Sylvian fissure. The cortical gray matter-lined cleft encasing the mass does not abut the ependymal surface of the lateral ventricle. Fat saturation sagittal T1 (D) shows signal dropout. Sagittal T1 post-gadolinium (E) shows no enhancement except for the venous component traversing through the lesion.
Diagnosis
Sylvian fissure lipoma with associated focal cortical dysplasia and venous malformation.
The differential diagnosis between intracranial fat-containing masses should be made with intracranial dermoid (scattered fat droplets in the subarachnoid space if ruptured), intracranial teratoma, and lipomatous transformation of neoplasms such as primitive neuroectodermal tumor, ependymoma, or glioma. Thrombosed berry aneurysms (usually have a calcified rim or hemosiderin staining best seen in gradient echo and SWI sequences), while white epidermoids are rare and will show restricted diffusion. Intracranial lipomas are congenital lesions, usually asymptomatic; therefore, they are mostly diagnosed incidentally or after brain imaging due to related symptoms such as headache, epilepsy, psychomotor retardation, or cranial nerve deficits.1
Discussion
Intracranial lipomas are congenital lesions and usually asymptomatic; therefore, they are usually diagnosed incidentally or after brain imaging due to issues such as headache, epilepsy, psychomotor retardation, or cranial nerve deficits.1
Intracranial lipomas are rare lesions with an incidence of 0.1-1.7% of all intracranial tumors, mostly located in the midline.2 Regarding their location, intracranial lipomas can be found anywhere; nevertheless, the most frequently reported sites are: 50% pericallosal with associated agenesis or dysgenesis of the corpus callosum in half of cases and 25% in the quadrigeminal plate cistern with associated underdevelopment of the inferior colliculus. Suprasellar cistern lipomas are encountered in 15% of cases, while 10% are found in the cerebellopontine angle, with the facial and vestibulocochlear nerves often coursing through the lesion. Sylvian fissure lipomas are rare, accounting for only 5% of cases, and choroid plexus lipomas are even rarer.3
Among the various theories proposed to explain the development of intracranial lipomas, the most widely accepted is the persistence and maldifferentiation of the meninx primitiva , a precursor of the subarachnoid space. This theory accounts for the typical subarachnoid location, associated parenchymal anomalies, and traversing vascular structures observed in such cases.4, 5
Various adjacent brain malformations are reported in 55% of cases with intracranial lipoma, with 36% of them showing traversing nerves and intracranial vessels through the lesion, best seen on high-resolution sequences.6 The most common associated malformations of the CNS in such a setting are agenesis or dysgenesis of the corpus callosum, absence of the septum pellucidum, encephalocele, myelomeningocele, vermis hypoplasia, cranium bifidum, spina bifida, and cortical dysplasia, as in this case.7
Imaging findings on both CT and MRI of intracranial lipomas reveal a mass of fat attenuation and signal intensity. Lipomas do not enhance. They have a lobulated appearance surrounded by a chemical shift artifact. They appear hyperintense in T1 and T2, show low signal intensity in the fat-saturation sequences, and produce blooming due to susceptibility artifact.8
In most cases, intracranial lipomas are asymptomatic, and no treatment is required even when associated with symptomatic malformations such as callosal dysgenesis. Local tumor growth is thought to be directly related to the onset or worsening of symptoms.
The main goal of surgery is adequate decompression. Because of their vascular characteristics and adhesion to surrounding parenchyma, resection attempts have sometimes led to little benefit and high morbidity; therefore, in most cases, only partial removal is carried out.1, 9, 10
Intracranial lipomas are slow-growing tumors with a favorable outcome. If the lesion is diagnosed incidentally, the patient should be scheduled for follow-up. Seizures or hydrocephalus must be treated if present.1, 9
Conclusion
Sylvian fissure lipomas are rare congenital lesions, asymptomatic in most cases, and therefore discovered incidentally. Association with other coexisting abnormalities, such as focal cortical dysplasia or venous malformations, is even rarer, as in this case. Despite limitations to completely remove such lesions, decompressive surgery is the mainstay of treatment in case of symptoms related to local tumor growth. The patient was scheduled for a follow-up assessment.
References
Citation
Gjonbrataj E, Hoti A, Stafa A, Rroji A.Sylvian Fissure Lipoma with Associated Focal Cortical Dysplasia and Venous Malformation. Appl Radiol. 2025;
doi:10.37549/AR-D-25-0100
November 1, 2025