Subtle Medical receives CE Mark approval and FDA clearance for SubtlePET™
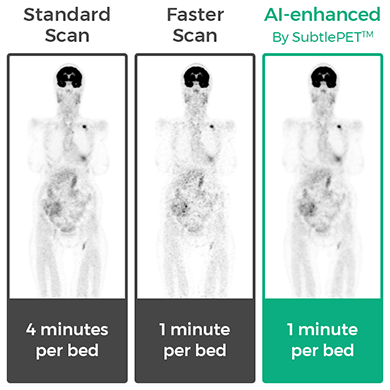 Subtle Medical of Menlo Park, CA, announced that SubtlePET™, an artificial intelligence (AI)-powered software program designed to increase positron electron tomography (PET) efficiency has received both CE Mark approval and 510(k) clearance from the U.S. Food and Drug Administration.
Subtle Medical of Menlo Park, CA, announced that SubtlePET™, an artificial intelligence (AI)-powered software program designed to increase positron electron tomography (PET) efficiency has received both CE Mark approval and 510(k) clearance from the U.S. Food and Drug Administration.
The company said that by focusing the SubtlePET AI platform on faster image acquisition, it is able to dramatically increase PET scan efficiency by significantly reduces a patient’s time in a PET scanner. The shorter scan time can also help improve the image quality of noise images.
SubtlePET technology utilizes deep learning algorithms that integrate seamlessly with any PET scanner and picture archive and communications system (PACS) to enhance images during acquisition without any interruption or alteration in the imaging specialists’ workflow.
A second product currently undergoing clinical evaluation is SubtleMR™, which allows imaging centers to significantly accelerate magnetic resonance imaging (MRI) scans.
Citation
Subtle Medical receives CE Mark approval and FDA clearance for SubtlePET™. Appl Radiol.
January 7, 2019