Sponge Lung: Pulmonary edema superimposed on emphysema
Images



CASE SUMMARY
A 54-year-old male with known history of emphysema, coronary artery disease, and congestive heart failure presented with chest pain and shortness of breath of three days’ duration. Physical exam is positive for increased work of breathing, diffuse crackles most prominent at the bases, and overall decreased breath sounds. The patient was afebrile with a normal white blood cell count. He was admitted and treatment begun for COPD exacerbation. Shortly after admission, the patient had rapid desaturation requiring treatment with a non-rebreather mask. Imaging was obtained for further evaluation. The patient’s B-type Natriuretic Peptide (BNP was 697 (BNP levels above 600 pg/mL indicate moderate heart failure).
IMAGING FINDINGS
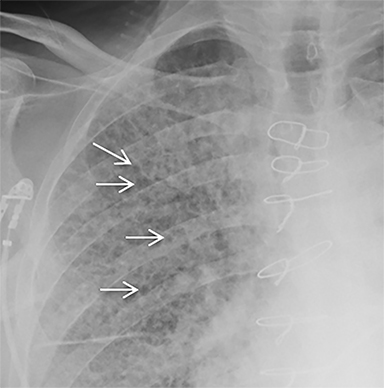
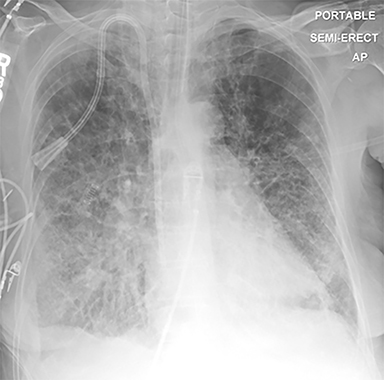
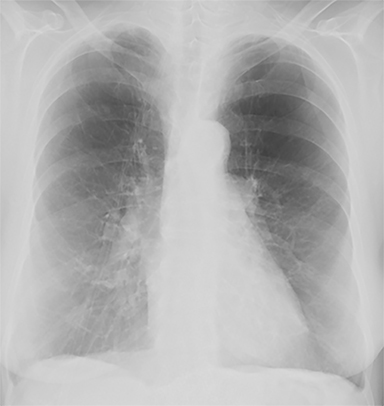
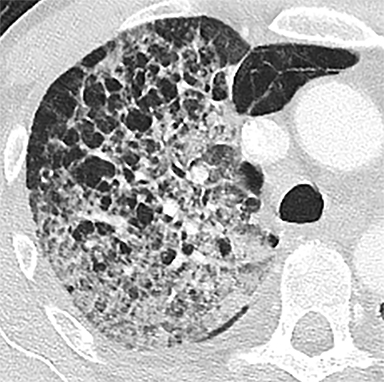
The patient’s imaging findings were consistent with “Sponge Lung”, a novel term and appearance not previously described in the radiologic literature. “Sponge Lung” describes the appearance of scattered centrilobular lucencies surrounded by reticular and alveolar opacities, creating the appearance of a sponge on radiographs and CT. Radiographically, this appears as bilateral coarse reticular and heterogeneous alveolar opacities with scattered rounded lucencies in a “mesh-like” pattern, resembling a sponge (Figure 1). On CT, this appears as diffuse, bilateral, smooth, interlobular septal thickening, and ground-glass opacities on a background of innumerable scattered centrilobular lucencies (Figure 2).
DIAGNOSIS
Pulmonary edema superimposed on background centrilobular emphysema
DISCUSSION
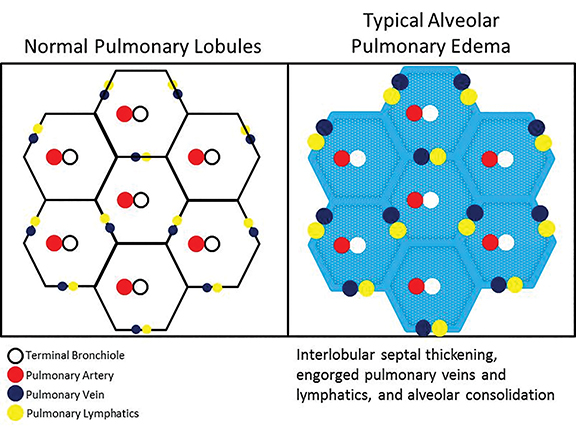
Classic radiologic patterns of pulmonary edema are rarely observed in the setting of background lung parenchymal disease with architectural distortion, particularly in the case of emphysema. Emphysema is defined by chronic abnormal inflammatory cell response resulting in destruction of small airway and alveolar walls, with coalescent destructive lesions producing larger cavities resulting in bullous disease.1 This alveolar destruction results in lung parenchymal distortion, making superimposed processes, particularly pulmonary edema, difficult to accurately identify. Though not comprehensively described in existing literature, the notion that radiologic evidence of heart failure is influenced by the presence of emphysema has been recognized. In particular, asymmetric, regional, and reticular patterns of pulmonary edema are commonplace in those with emphysema.2,3
Regional patterns are observed because edema can only physiologically occur in the presence of an intact parenchymal bed. In the case of emphysema, there is often apical predominant destruction of lung tissue, confining edema in a regional distribution to the lower lung zones.4 Reticular patterns of edema, radiographically indicated by the presence of Kerley A and Kerley B lines, have also been described as occurring with increasing frequency in the presence of emphysema.5 This is attributed to impairment of lymphatic flow in the chronically diseased lung with decreased compliance and elasticity, resulting in stasis and engorgement of the lymphatic network even in the absence of significantly elevated pulmonary venous pressures. Therefore, even a slight increase in pulmonary venous pressures, much less than needed in a non-diseased lung, can tip the patient into interstitial edema with a reticular pattern. Nodular patterns of edema have also been described with underlying emphysema, attributed to the impairment of collateral ventilation through the pores of Kohn and channels of Lambert.6
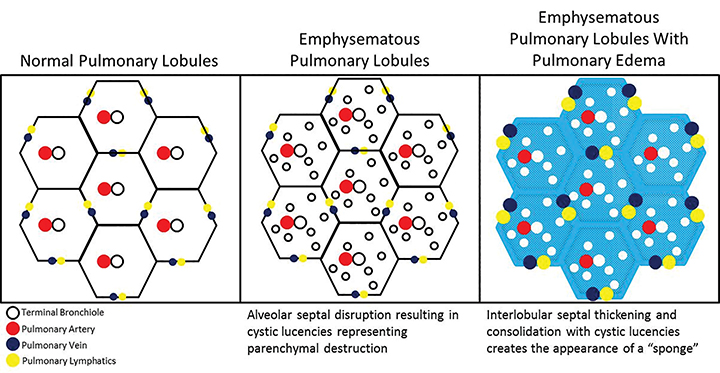
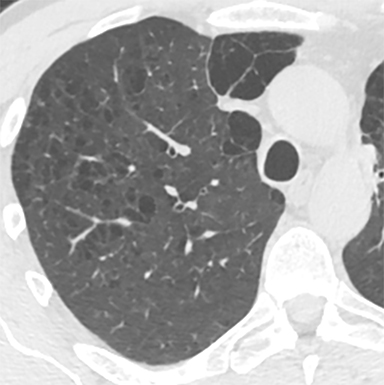
In our experience, however, a new radiologic pattern of pulmonary edema superimposed on emphysema is often seen, one that we term Sponge Lung due to its characteristic appearance, not previously described in the radiologic literature. As discussed, emphysema leads to distortion of the lung parenchyma due to alveolar destruction, specifically creating rounded lucencies within the secondary pulmonary lobule (Figures 3-5). When a superimposed diffuse interstitial and alveolar process then occurs, it accentuates these lucencies within the secondary pulmonary lobule amongst surrounding opacity creating the appearance of a sponge on radiographs and CT.
The development of regional airspace disease with scattered areas of radiolucency in a patient with centrilobular emphysema has previously been described in patients with pneumonia and has been termed a “Swiss Cheese” appearance, which describes non-uniformly perforated emphysematous lung tissue amidst dense consolidation.7-9 However, both the pathophysiology and imaging of Sponge Lung shows a different appearance. Rather than the dense consolidation manifest in the “Swiss Cheese” appearance, Sponge Lung is characterized predominantly by interlobular septal thickening and ground-glass opacity.
Recognizing the appearance of pulmonary edema superimposed on emphysema has many clinical implications. First, the coexistence of heart disease and emphysema is not uncommon. In addition, it is widely known that COPD delays the diagnosis of CHF. When patients without known respiratory disease complain of dyspnea or fatigue during exercise, they routinely undergo noninvasive cardiac imaging that establishes the diagnosis of heart failure when it demonstrates left ventricular (LV) dysfunction. When patients with stable COPD complain of dyspnea or fatigue during exercise, however, these symptoms are often attributed to COPD, and noninvasive cardiac imaging is routinely performed, leaving potential LV dysfunction undetected. Therefore, there is potential for radiologists to make significant contributions in a patient’s overall disposition and treatment by recognizing the appearance of pulmonary edema and findings of congestive failure superimposed on a background of emphysematous changes with important therapeutic implications.10
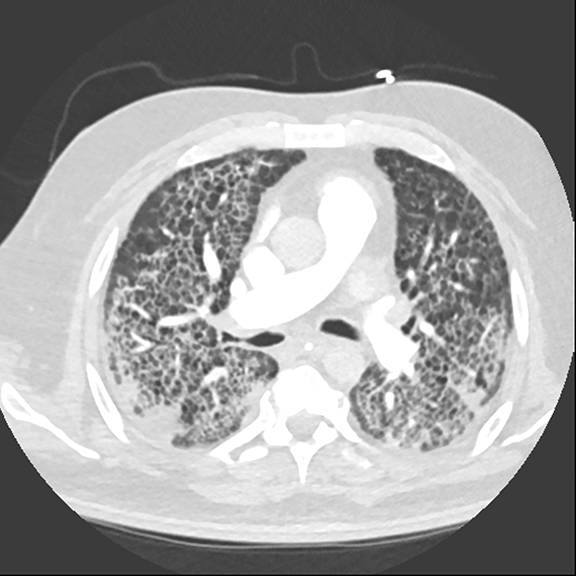
Recognition of Sponge Lung is important as CT findings are often mistaken for interstitial lung disease, potentially resulting in unnecessary biopsies and bronchoscopy, which in and of themselves carry significant procedural risk in patients who frequently have comorbid conditions. The reticular pattern observed radiographically is often misinterpreted as diffuse fibrosis, peribronchial fibrosis, honeycombing, suspected bronchiectasis, pleural-pulmonary scarring, or diffuse cystic lung disease.3 Sponge Lung is not specific for pulmonary edema and may represent any diffuse alveolar process superimposed on emphysema, including atypical infections, pulmonary hemorrhage, or non-cardiogenic causes of pulmonary edema. However, given that the most common etiology of a diffuse bilateral process in this population is pulmonary edema, the appearance of Sponge Lung should be interpreted as pulmonary edema superimposed on emphysema in the absence of clinical factors suggesting other possible etiologies, e.g. an immunocompromised patient (Figure 6).
CONCLUSION
Pulmonary edema superimposed on emphysema has been termed Sponge Lung due to its characteristic likeness to the appearance of a sponge. On radiographs, this appears as diffuse reticular and alveolar opacities with scattered rounded lucencies. On CT, this appears as smooth interlobular septal thickening and alveolar consolidation/ground-glass on a background of centrilobular lucencies.
Sponge Lung fittingly also describes the underlying physiology of pulmonary edema, i.e. lung parenchyma acting as a resevoir for transudative fluid, much like a sponge.
REFERENCES
- Mead J, Turner JM, Macklem PT, et al. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22(1):95-108.
- Milne EN, Bass H. Roentgenologic and functional analysis of combined chronic obstructive pulmonary disease and congestive cardiac failure. Invest Radiol. 1969;4(3):129-147.
- Hublitz UF, Shapiro JH. Atypical pulmonary patterns of congestive failure in chronic lung disease. The influence of pre-existing disease on the appearance and distribution of pulmonary edema. Radiology. 1969;93(5):995-1006.
- Rosenow EC III, Harrison CE. Congestive heart failure masquerading as primary pulmonary disease. Chest. 1970;58(1):28-36.
- Rigler LG, Suprenant EL. Pulmonary edema. Seminars in Roentgenol. 1967;2(1):33-47.
- Heard B. A pathologic study of emphysema of the lungs with chronic bronchitis. Thorax. 1958;13(2):136-149.
- Foster WL, Gimenez EI, Roubidoux MA, et al. The emphysemas: radiologic-pathologic correlations. Radiographics. 1993;13(2):311-328.
- Yudin A. Swiss cheese appearance. In: Metaphorical signs in computed tomography of chest and abdomen. Switzerland: Springer International Publishing; 2014:23.
- Parker MS, Rosado-de-Christenson ML, Abbott GF. Proximal acinar emphysema. In Teaching atlas of chest imaging. New York: Thieme; 2005:145.
- Rutten FH, Cramer MJ, Lammers JW, et al. Heart failure and chronic obstructive pulmonary disease: An ignored combination? Eur J of Heart Fail. 2006;8(7):706-711.
Citation
SJ K, M H, J B.Sponge Lung: Pulmonary edema superimposed on emphysema. Appl Radiol. 2016; (10):31-34.
October 15, 2016