SNMMI 2019: Radiopharmaceutical monitors rheumatoid arthritis progression
Intravenous administration of technetium-99m (99mTc) tilmanocept, a radiopharmaceutical imaging agent, shows promise as a safe and effective means of monitoring rheumatoid arthritis (RA) progression, according to a study presented in June at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) in Anaheim, CA. The results of this first-in-human phase I/phase II clinical study generated excitement among attendees, as no reliable, noninvasive way currently exists to directly monitor joint inflammation.
Rheumatoid arthritis is caused in part by the release of pro-inflammatory cytokines and chemokines by activated macrophages,immune system cells involved in detecting and destroying harmful organisms.99mTc tilmanocept (TCT) binds to the macrophage mannose receptor CD206, which is highly expressed on activated macrophages in RA.
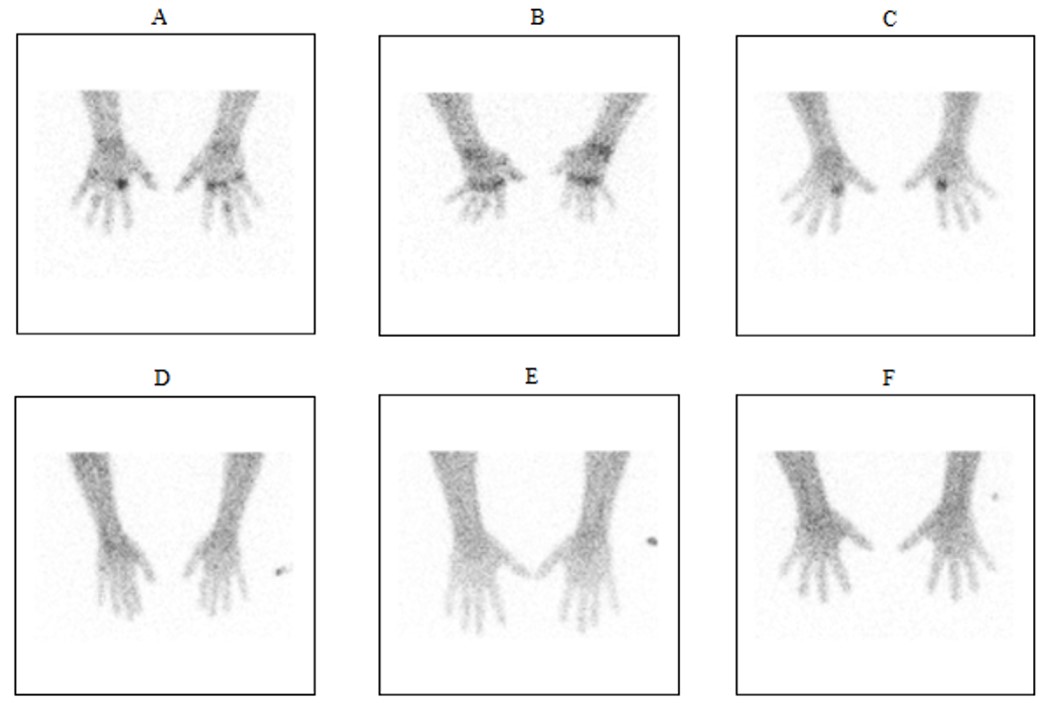
A comparison of Tc-99m tilmanocept radiopharmaceutical uptake on planar imaging in subjects with active RA (A: 200 μg tilmanocept/10 mCi 99mTc; B-c: 400 μg tilmanocept/10 mCi 99mTc) versus healthy controls (D-F: 400 μg tilmanocept/10 mCi 99mTc) at three hours post-IV administration. Images courtesy of A. Kardan et al. University Hospitals/Case Western Reserve University, Cleveland, OH.
“Intravenous administration of TCT provides a novel, noninvasive molecular imaging marker that demonstrates joint-specific, CD206-expressing synovial macrophage involvement,” said lead author Arash Kardan, MD, a radiologist in the nuclear medicine division at University Hospitals of Cleveland and an associate professor of radiology at Case Western Reserve University, also in Cleveland. “It reveals potentially significant qualitative and quantitative immunodiognostic information regarding the distribution and severity of active disease involvement in RA patients.”
“TCT administration in both RA patients and healthy controls presents initial safety and tolerability findings, as well as a powered determination of an optimal clinical dose and time point for imaging,” he said.
Sponsored by Navidea Biopharmaceuticals (Dublin, OH), the study focused on 33 patients with active RA and six healthy volunteers. They were divided into 11 groups who received various dose combinations of IV administered99mTc at 1, 5, and 10 mCi, radiolabeled with 50, 200, or 400 μg of tilmanocept. None of the study subjects experienced any adverse drug reactions.
Standard gamma camera whole-body planar imaging, as well as anteroposterior (AP) spot views of the hands and wrists, were performed one and three hours following injection. Six RA patients and the 6 healthy controls underwent whole-body planar scans for radiodosimetry assessment and pharmacokinetic analysis.
The researchers performed a quantitative assessment of count activity in individual joint spaces of the hands and wrists. Count activity was extracted by placing separate regions of interest on one- and three-planar anterior images. The data were used to model the optimal time point and dose for detecting TCT uptakes in involved RA joints.
Dr. Kardan said planar imaging showed TCT localizing specifically to inflamed joints but not to healthy joints. Study data analysis revealed an optimal mass dose of 134 μg of tilmanocept radiolabeled with 10 mCi99mTc with imaging taking place 60 to 360 minutes after injection. He recommended a mass dose of 150 μg with the same imaging time frame for future research and use.
“These results provide the foundation for a noninvasive method to monitor disease activity in machrophage-driven inflamed joints with RA, including evaluation of responses to therapy,” said Dr. Kardan. “It may help to guide the most effective therapeutic strategy for RA patients, including when to initiate therapy. This could be of great benefit for the millions of individuals in the world who have this debilitating and painful disease.”
REFERENCE
- Kardan A, Kissling A, Hartings C, et al. A phase I/phase II study of intravenously (IV) administered Tc 99m tilmanocept (TCT) to determine safety, tolerability, optimal clinical dose selection, and imaging timepoint in patients clinically diagnosed with rheumatoid arthritis (RA).J Nucl Med. 2019;60(Suppl 1):89.