Sinonasal neoplasms: Update on classification, imaging features, and management
Images






Imaging of the sinonasal (SN) cavities is one of the most frequently performed examinations, primarily for evaluation of presumed inflammatory disease. Although SN neoplasms are relatively uncommon compared to other head and neck tumors, radiologists are likely to encounter these lesions because of the frequency of sinus imaging. Many of these tumors have nonspecific presenting symptoms and imaging features; however, some distinguishing features can help narrow the differential diagnosis. The SN cavities are one of the most histologically diverse areas of the body, with a large variety of neoplasms occurring in the region. Our goal is to discuss some of the more common entities and their characteristic features, as well as some less common lesions that are of greater importance. We also briefly discuss imaging techniques, tumor presentation, classification and management.
Imaging
Many patients with SN neoplasms are initially imaged with computed tomography (CT), as the presenting symptoms often mimic inflammatory disease. Although CT is not the best modality for characterizing neoplasms and mapping extent of disease, CT can help define the site of origin, depict bony remodeling vs. bony destruction, and detect internal calcifications and tumor matrix. Magnetic resonance imaging (MRI) has superior soft-tissue differentiation compared to CT. MRI is useful for mapping tumor extent, differentiating tumor from obstructed secretions, delineating the internal character of the tumor (cellularity, vascularity, architecture), and evaluating for perineural tumor spread (PNTS). Nodal involvement is infrequent with SN malignancies, but both CT and MRI are helpful for detecting retropharyngeal and level II nodes, those most frequently involved. Positron emission tomography (PET) is not utilized in many cases, but can aid in detection of nodal disease and distant metastases. It is also helpful for directing biopsy, evaluating response to therapy, and detecting residual or recurrent disease in the previously treated patient.
Most SN tumors have nonspecific imaging features, often requiring pathologic diagnosis. While differentiating one neoplasm from another can be difficult, imaging can often help differentiate benign from malignant lesions. For example, findings typically associated with benign tumors include bony remodeling or thickening of the adjacent bone, whereas malignant tumors tend to destroy bone. Additional findings suggestive of malignancy include ill-defined tumor margins, PNTS, and presence of cervical lymphadenopathy.
Clinical presentation
Unfortunately, many SN neoplasms present with nonspecific signs and symptoms that mimic rhinosinusitis. These include unilateral nasal obstruction, nasal discharge and headache. This frequently delays diagnosis as the patient is initially treated for inflammatory disease. Vascular tumors may present with epistaxis in addition to nasal obstruction. More advanced lesions may produce symptoms such as anosmia, visual disturbance, diplopia, cranial neuropathy, facial swelling, facial pain, or epiphoria.
Tumor classification
The World Health Organization (WHO) classification system divides SN tumors into categories based tissue of origin and benignity vs. malignancy.1 The histologic categories of neoplasms include epithelial lesions, soft tissue tumors, tumors of bone and cartilage, hematolymphoid tumors, neuroectodermal tumors, germ cells tumors and secondary tumors. Further subdivision within each of these categories is based upon benignity vs. malignancy and tissue type; ie, salivary tissue, neuroendocrine tissue. This review will highlight the most common SN tumors, benign followed by malignant, and is organized in accordance with the WHO classification scheme. The complete WHO classification of SN tumors can be found online at http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/index.php.
Benign neoplasms
Epithelial tumors
Benign epithelial tumors include papillomas and salivary gland-type adenomas, the most common of which is the pleomorphic adenoma (benign mixed tumor).
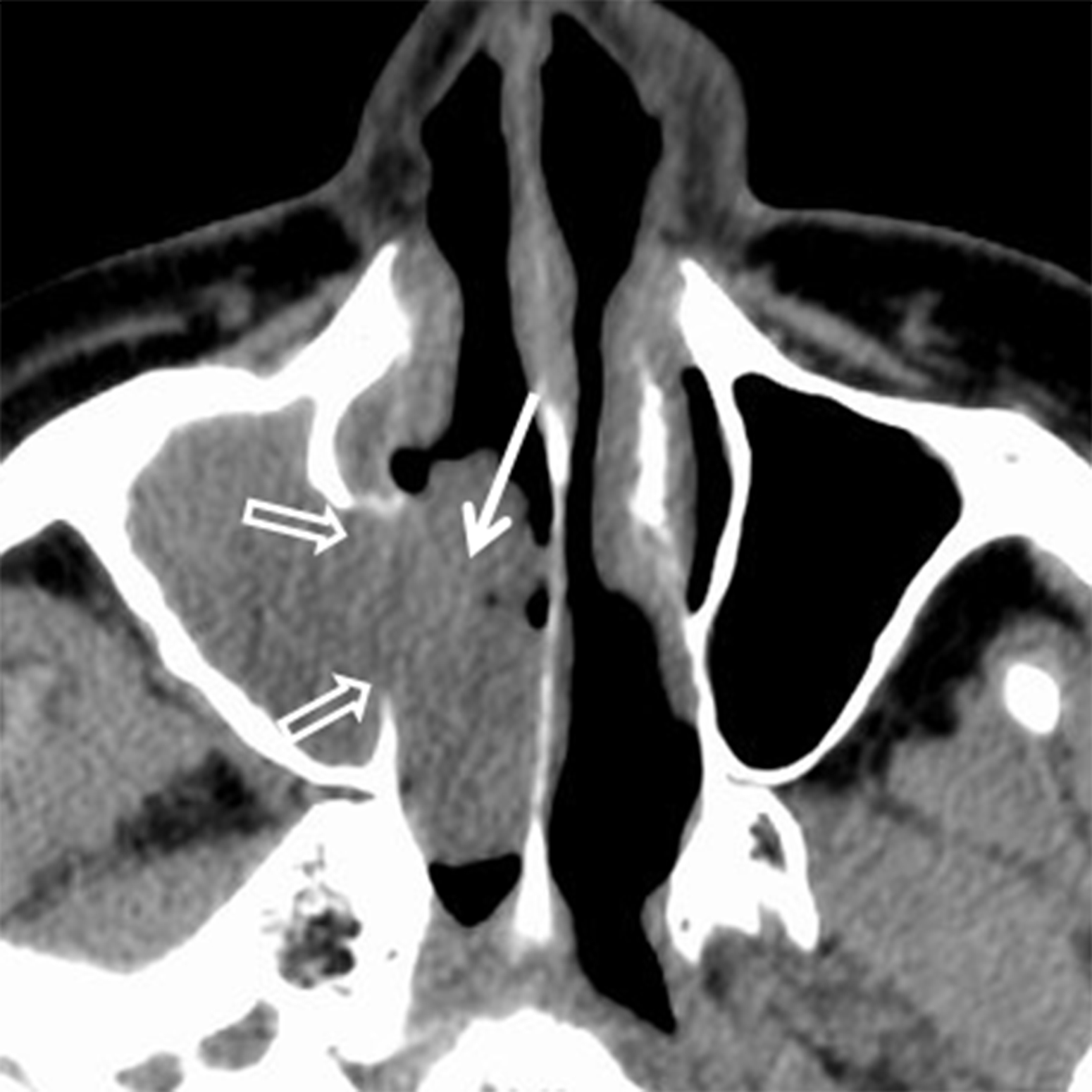
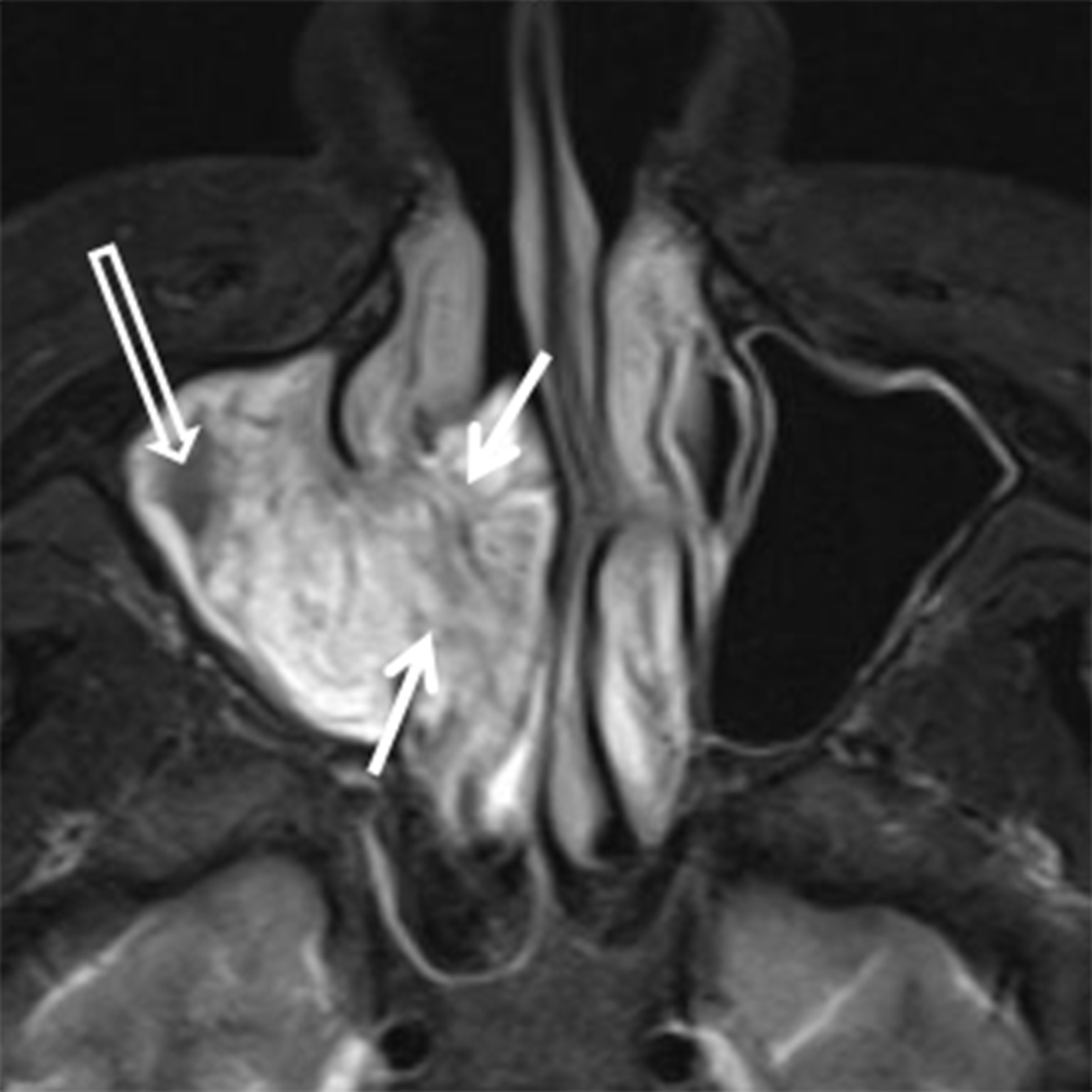
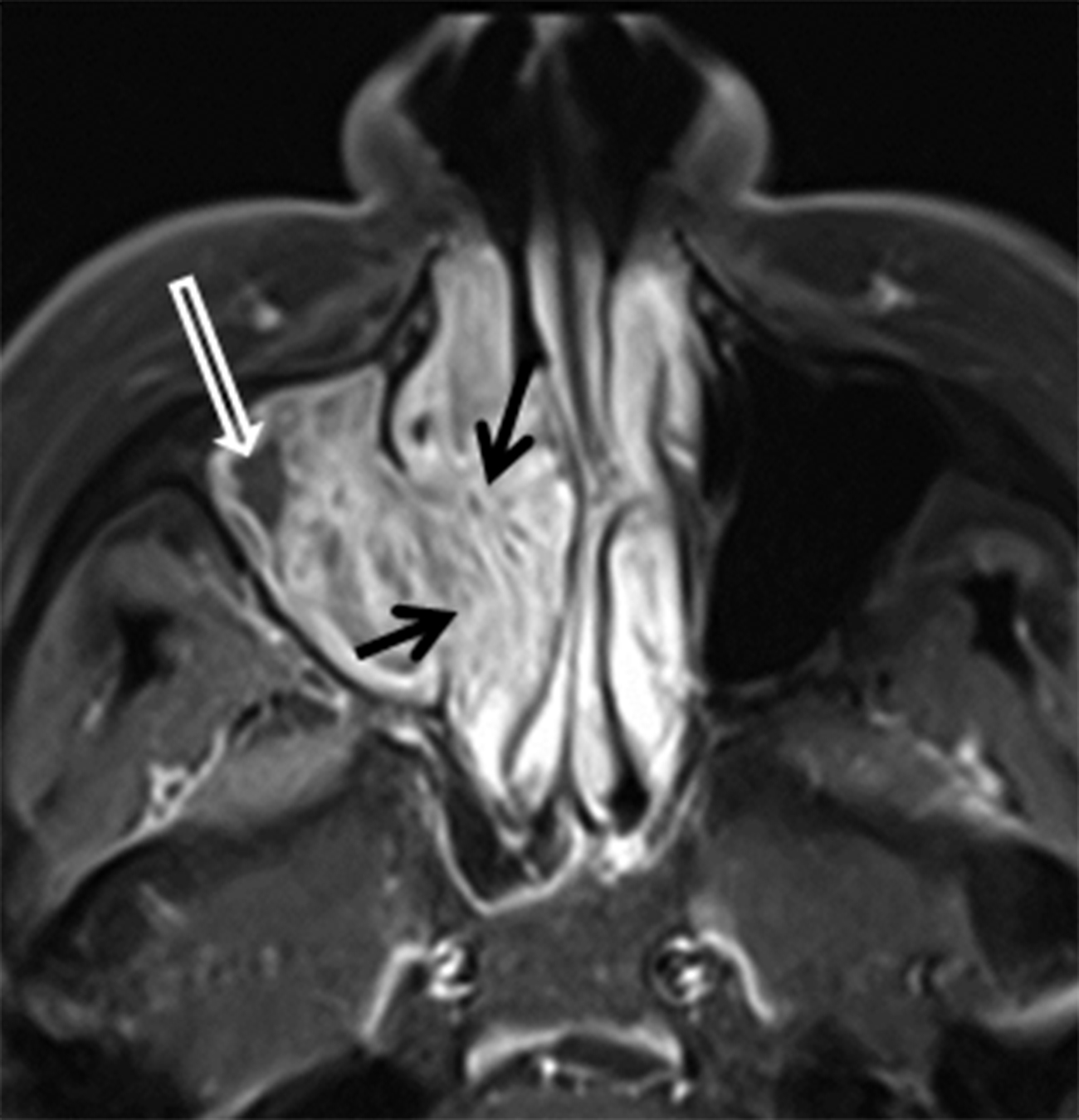
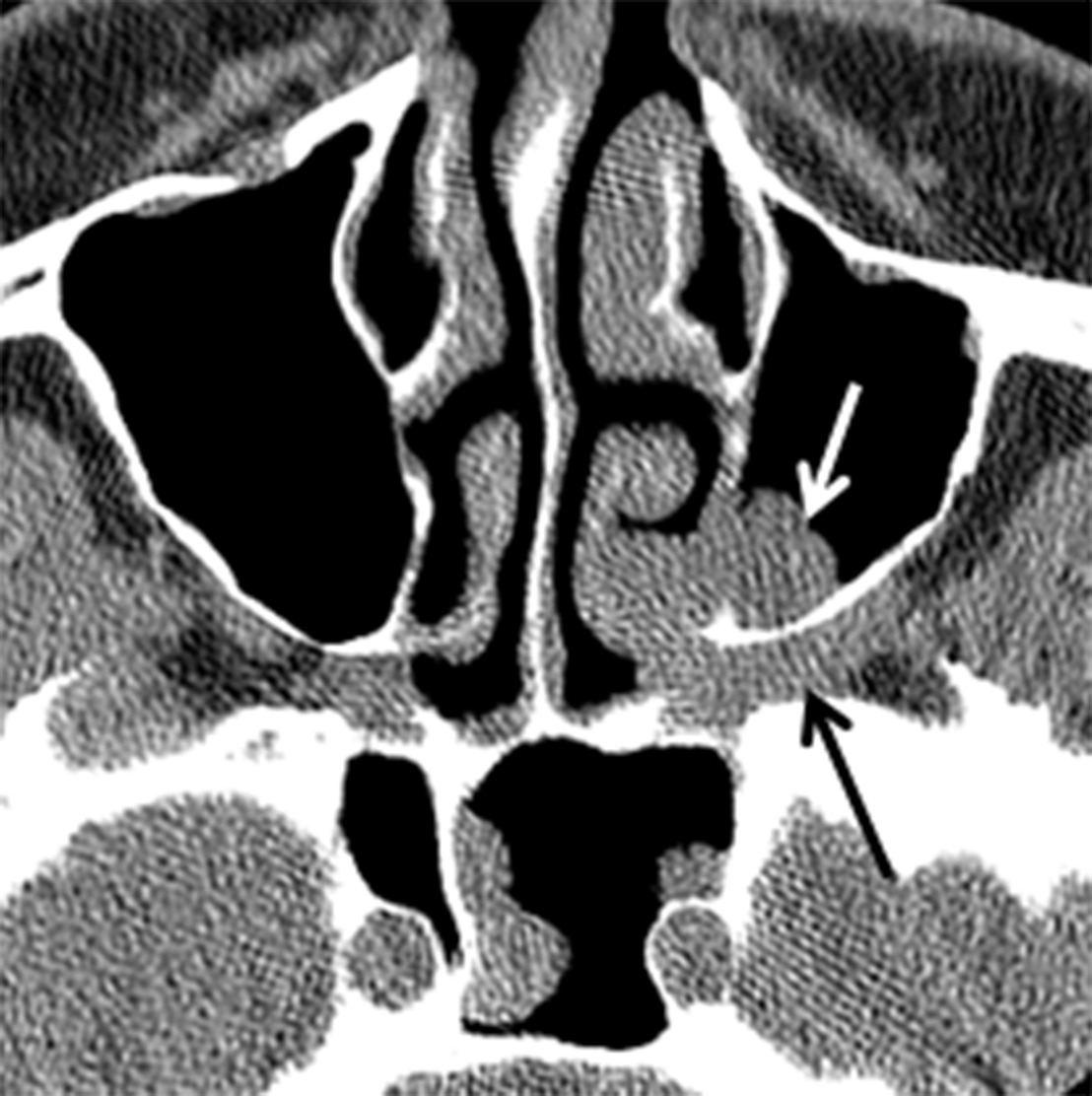
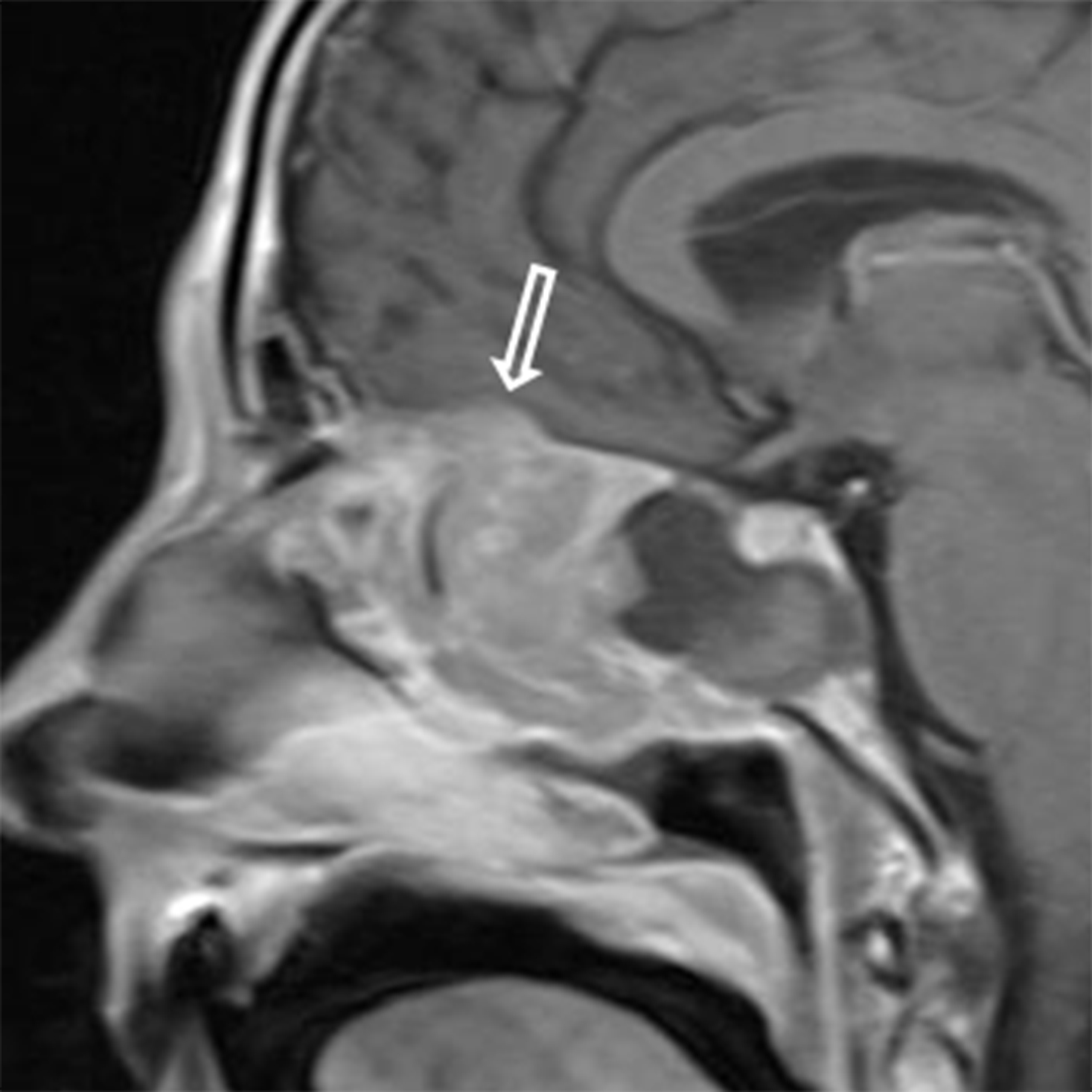
Papillomas are the most frequently encountered benign epithelial neoplasms, and the inverted papilloma (IPap) is the most common subtype. IPap is a benign but locally aggressive tumor with an estimated incidence of 0.6-1.5 per 100,000.2 The term “inverted” refers to the histology of the lesion, as IPap demonstrates an endophytic growth pattern. Usually presenting in the 5th through 7th decades, IPap afflicts men more commonly than women (M:F ≈3.5:1).2 Patients often present with unilateral nasal obstruction. IPap is associated with synchronous or metachronous malignancy, usually squamous cell carcinoma (SCCa), in 5-7% of cases.3 Human papilloma virus (HPV) has been implicated as a possible factor in the etiology of these lesions and certain types of HPV may increase the risk of developing malignancy within an otherwise benign IPap.4-5 The most common site of origin is along the lateral nasal wall near the middle meatus (Figure 1A). CT delineates bony remodeling and focal erosion and may show a bony strut or focal hyperostosis at the point of attachment of the lesion. It is important to report the site of attachment, as complete resection is required to prevent recurrence. The characteristic architecture of IPap is best shown on T2/STIR and post-gadolinium T1-weighted MR images. A convoluted, “cerebriform,” or columnar pattern is characteristic of IPap (Figures 1B,1C).6 Foci of necrosis or disruption of that architecture should raise suspicion for coexistent SCCa. Treatment is complete surgical excision as incomplete resection results in local recurrence.
Pleomorphic adenoma, also known as “benign mixed tumor,” arises from salivary rests in epithelial tissue, usually from the nasal septum. They are slow-growing lesions that remodel rather than destroy bone. They are generally seen in patients from 30-60 years of age with a female predominance.7 CT features of the lesion are nonspecific, but it can be useful for demonstrating bony remodeling. MRI classically demonstrates hyperintense T2 signal. Enhancement of BMT is variable and tends to be heterogeneous in larger lesions. In most cases, histology is required to confirm the diagnosis.
Soft-tissue tumors
Benign soft-tissue tumors of the SN cavities are rare and include nerve-sheath tumors, hemangioma and angiofibroma, myxoma, leiomyoma, and meningioma.
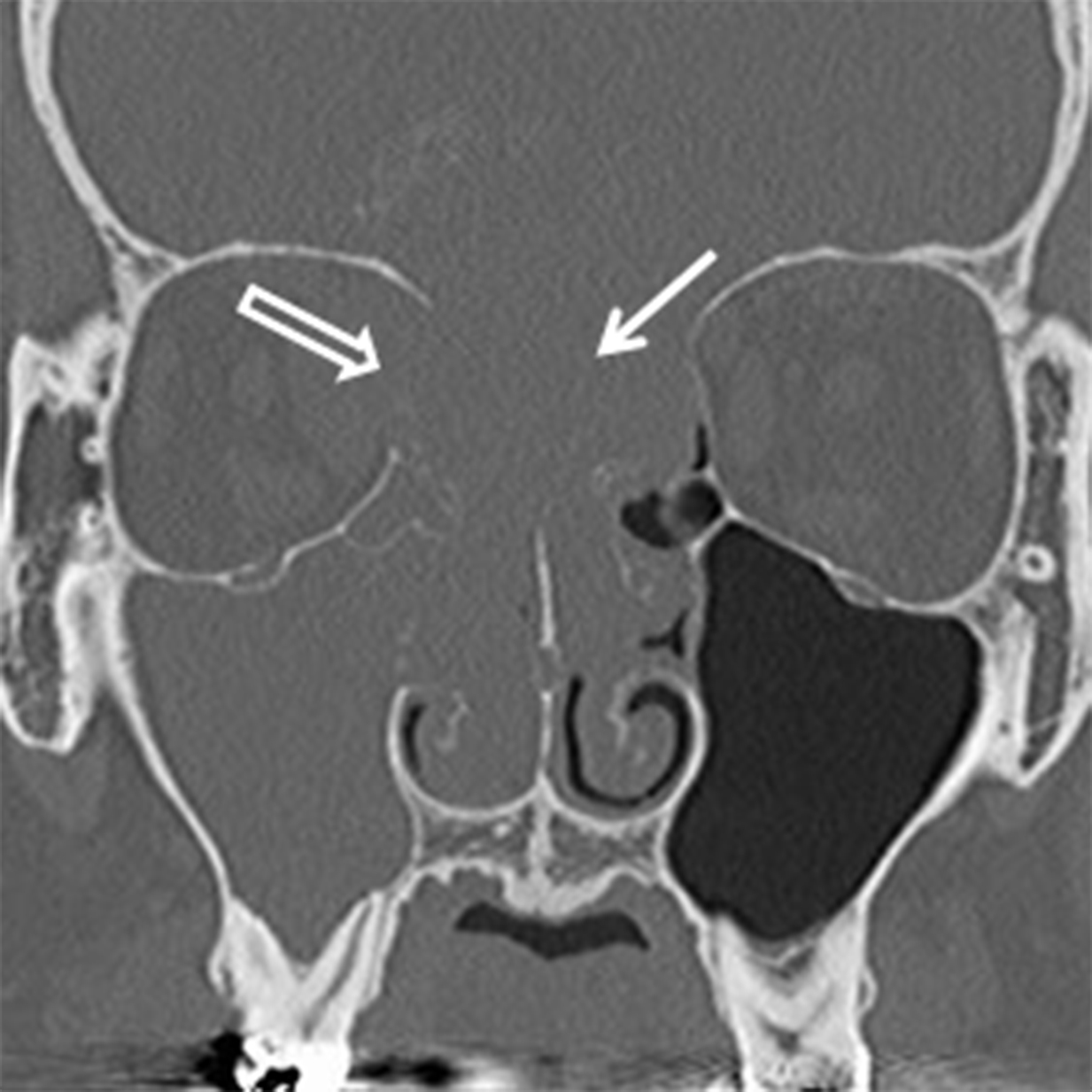
Neoplasms of nerve/sheath origin are relatively rare in the SN region. Neurofibromas arise from peripheral nerves and occur sporadically or in association with neurofibromatosis (NF) type 1. Schwannomas arise from the nerve sheath and are seen sporadically or in association with NF-2. There is no gender or race predominance, and these tumors can be seen at any age, although schwannomas are more common in the 30- to 50-year-old range.8-9 Transformation of these lesions into malignant peripheral nerve sheath tumors is rare, but is more common with neurofibroma than schwannoma.9 Imaging with CT demonstrates a well-defined mass with benign bone remodeling. These tumors are typically isodense to muscle, however some neurofibromas demonstrate low attenuation or an almost cystic appearance. T1W MR demonstrates intermediate signal in both lesions while T2W MR is more variable (Figure 2). A “bubbly” hyperintense pattern may be seen in schwannomas and large schwannomas develop intramural cysts. Contrast enhancement is variable, although schwannomas may demonstrate a whorled pattern of enhancement. Treatment for these tumors is surgical excision and prognosis is excellent.
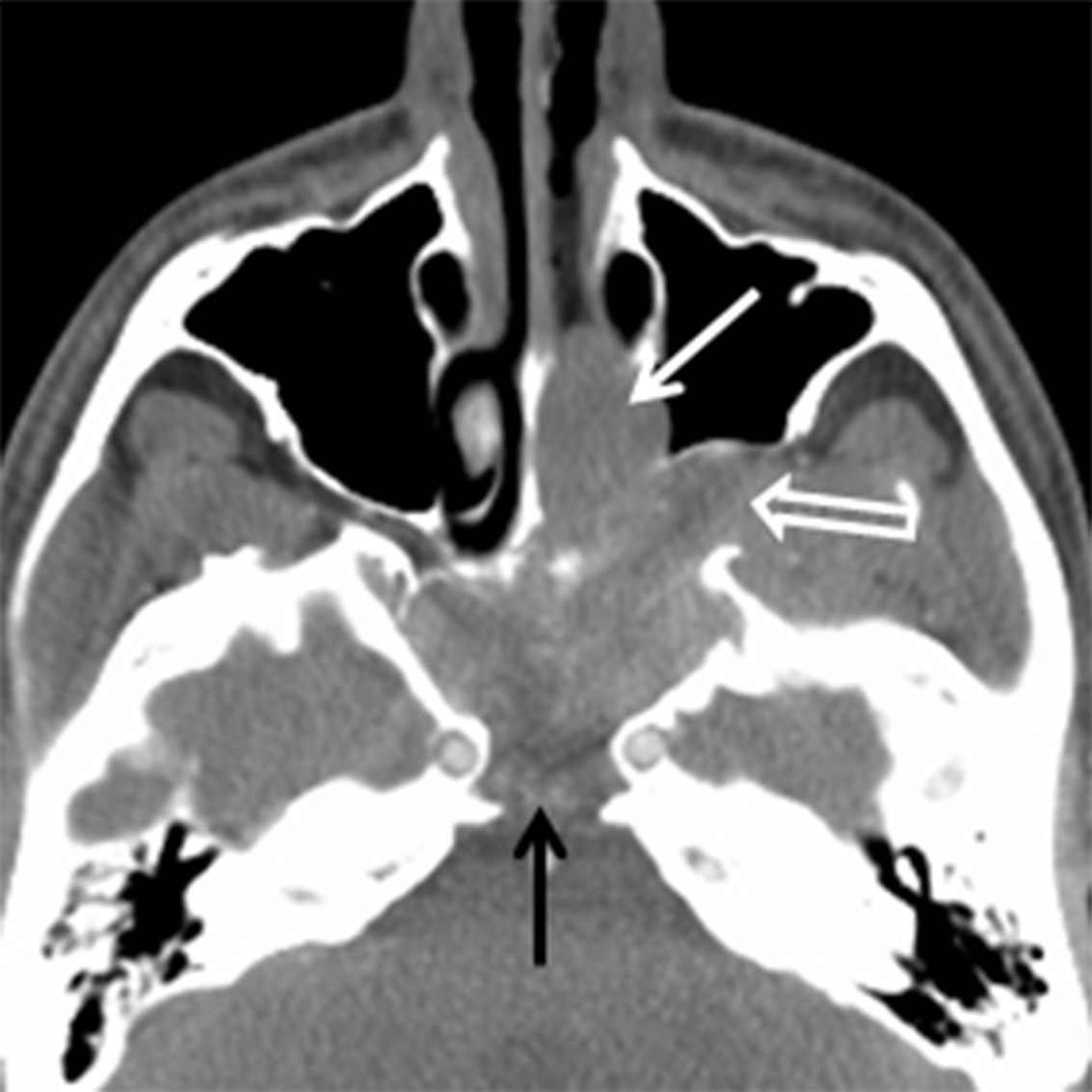
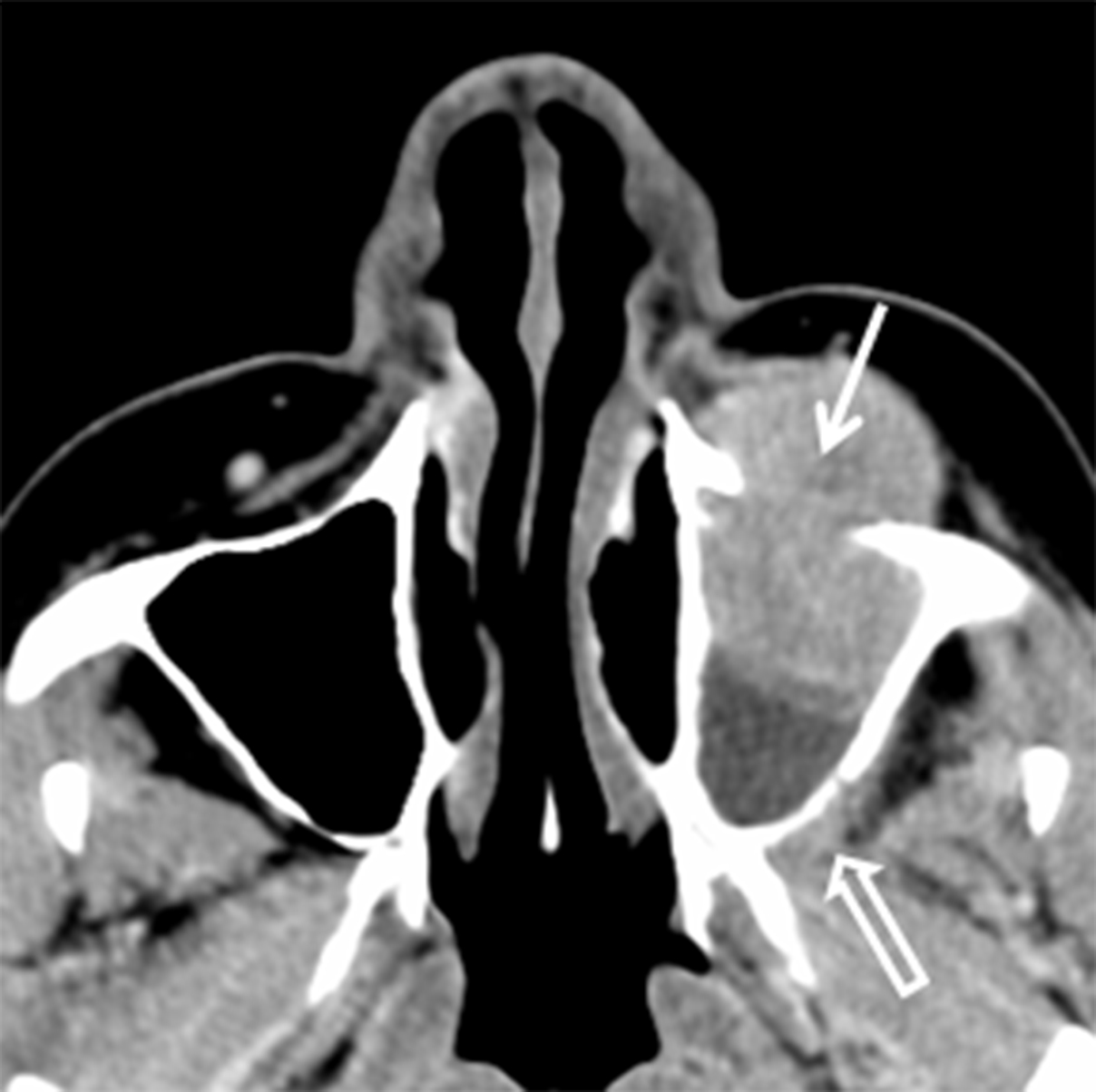
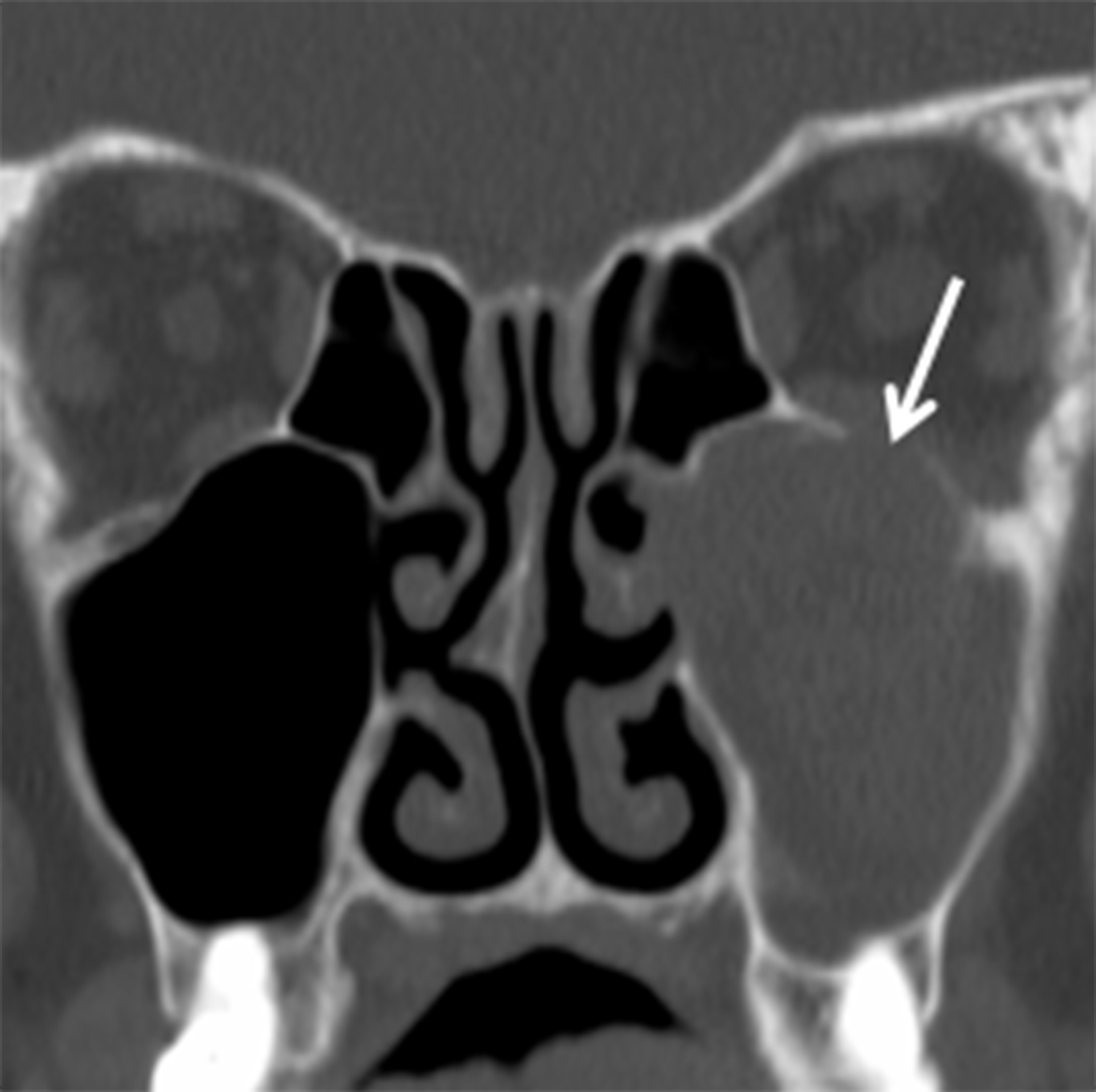
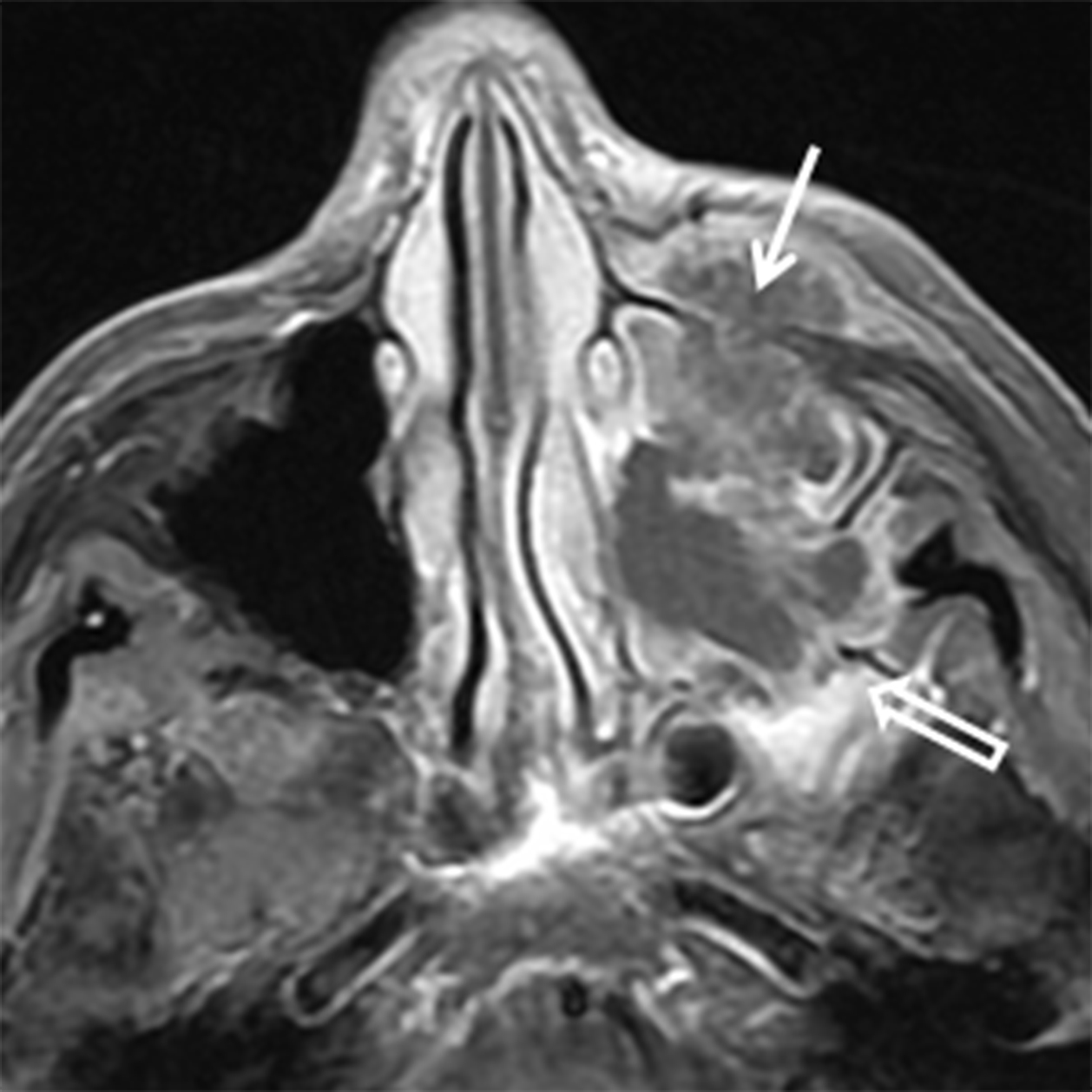
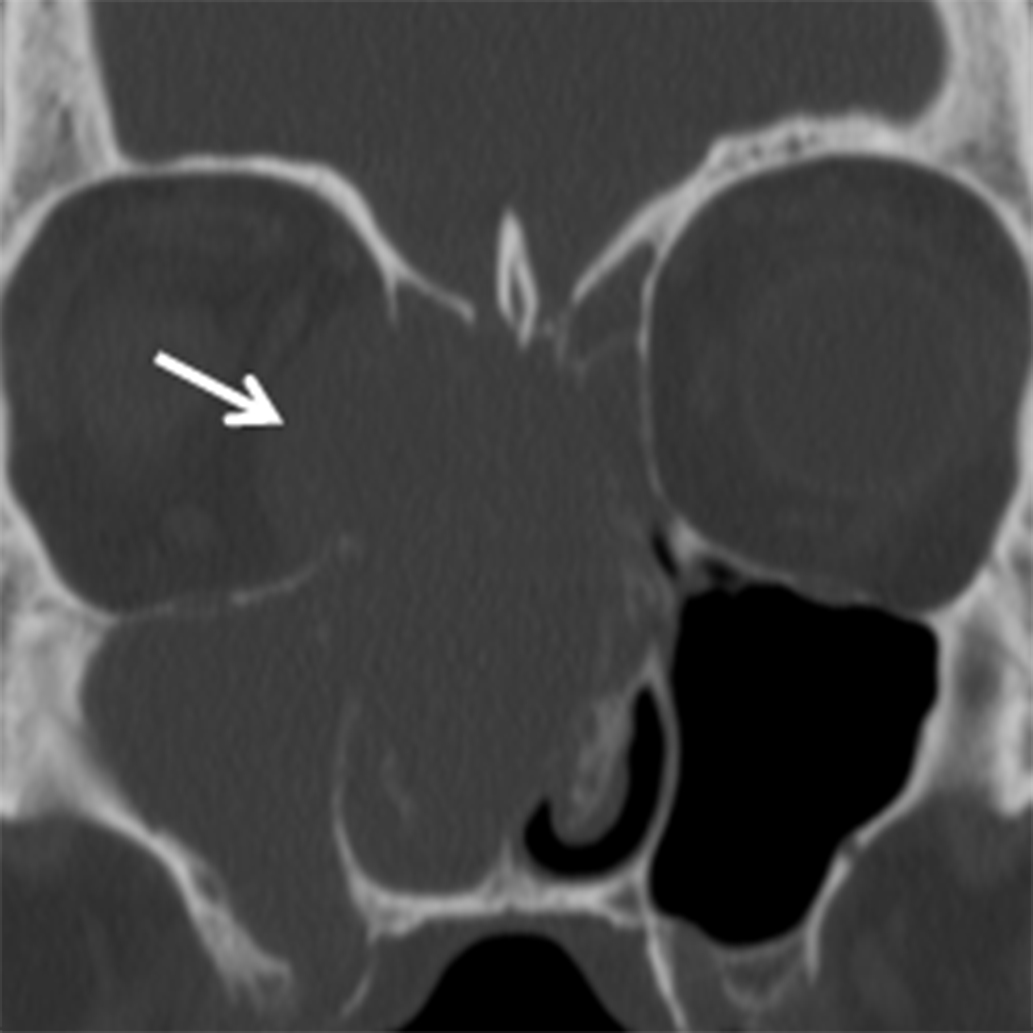
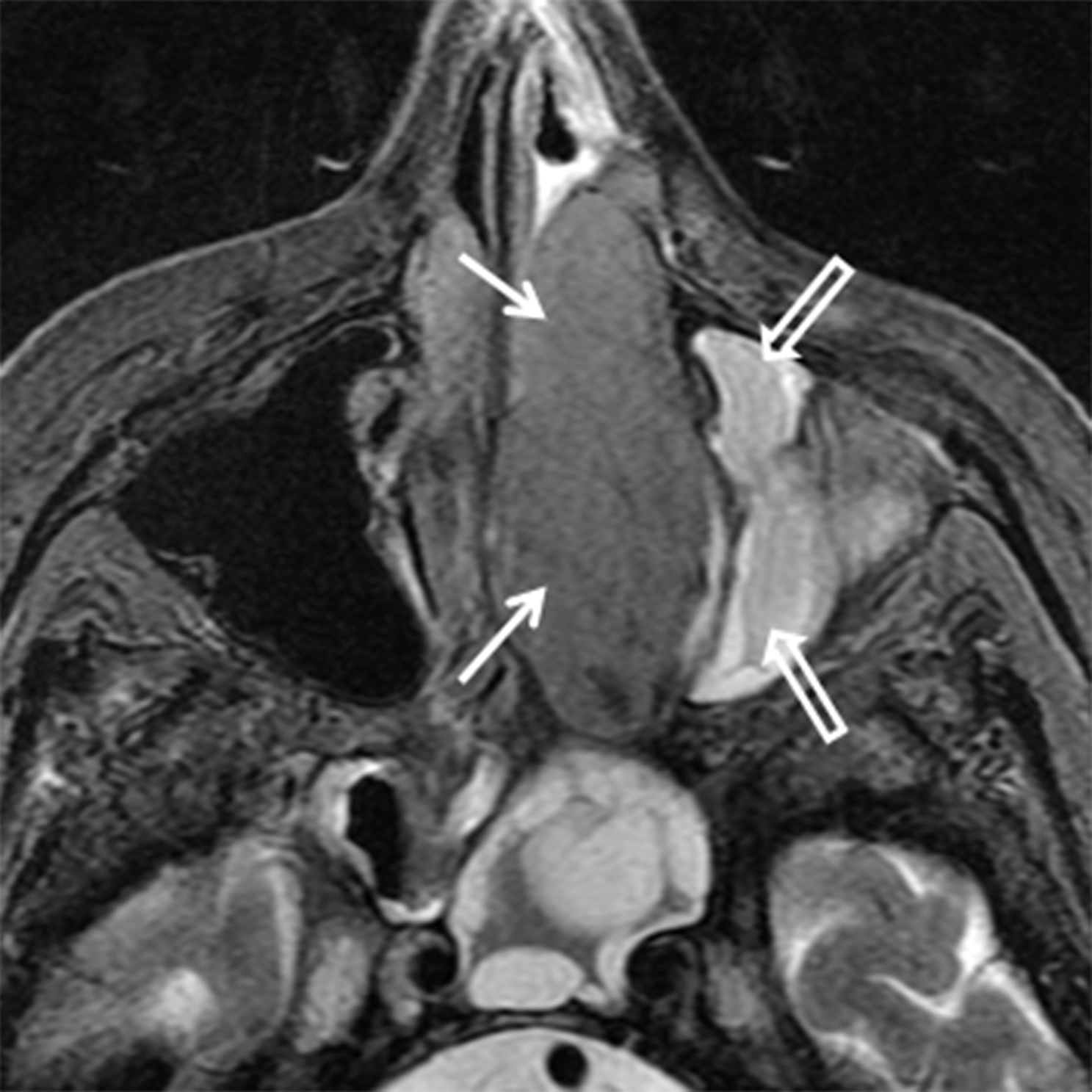
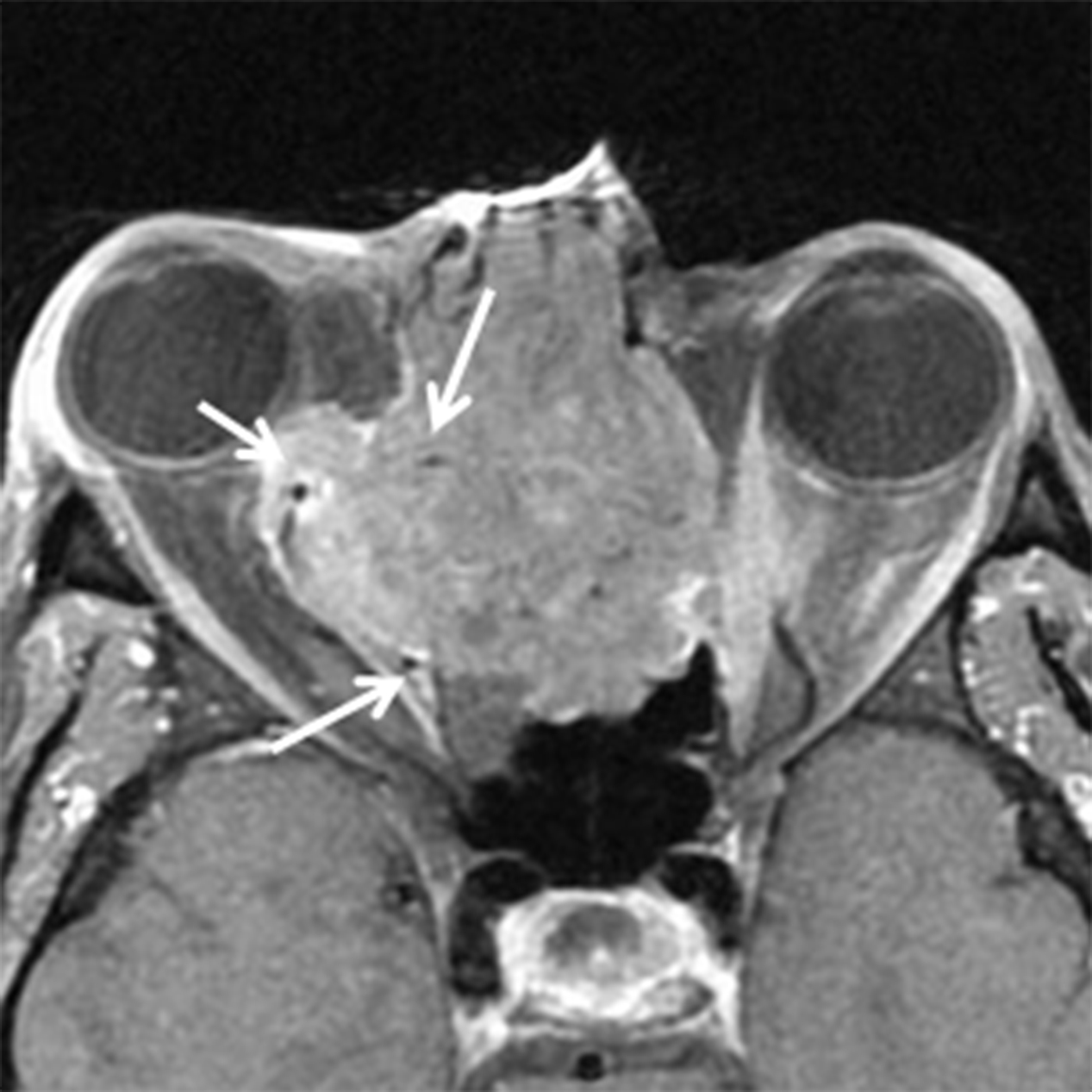
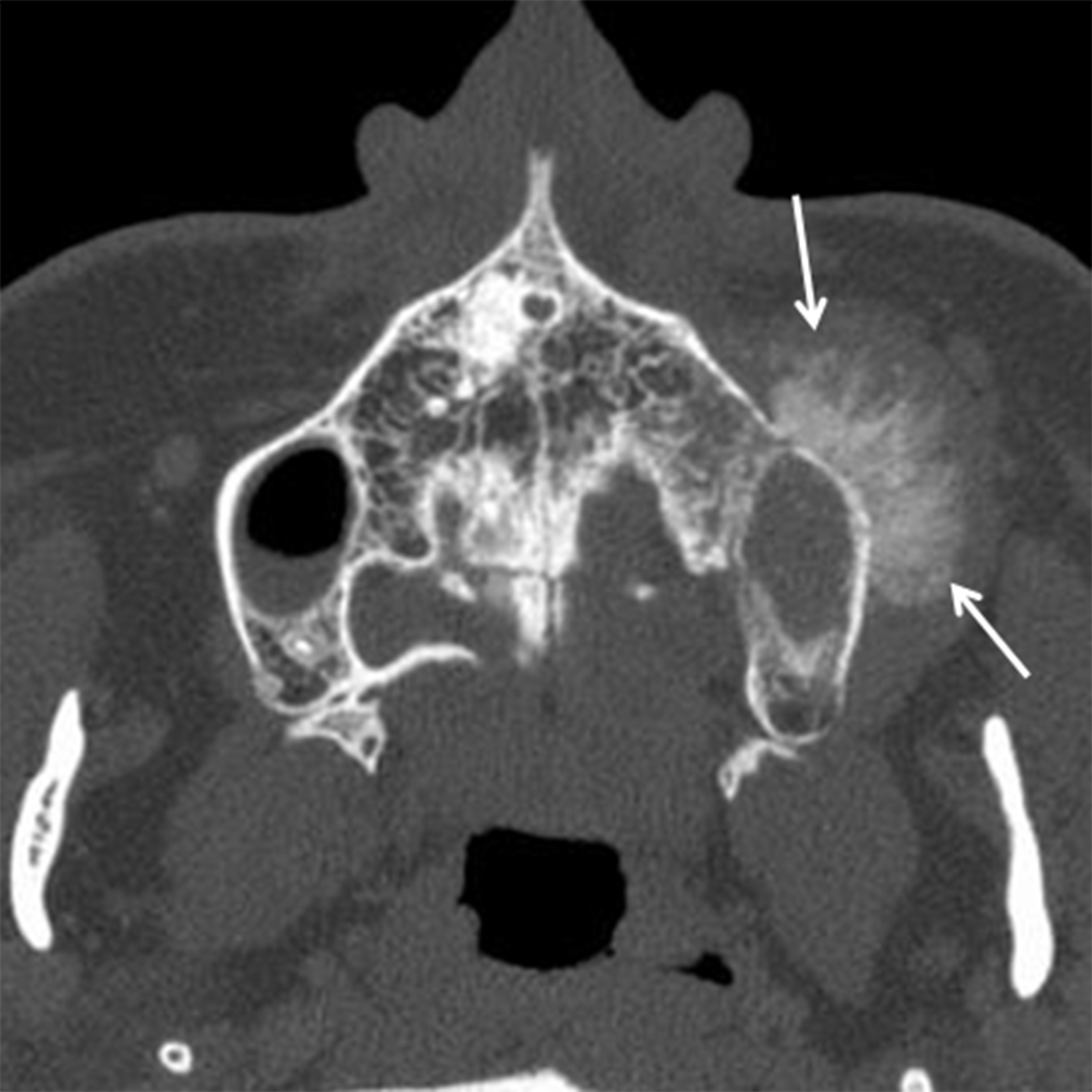
Juvenile angiofibroma (JAF) is a benign, highly vascular tumor of uncertain etiology with an incidence of 1 in 5000-60,000.10 JAF occurs in young males, typically between 10 and 25 years of age.10 If seen in a female, genetic mosaicism should be considered.11 In addition to unilateral nasal obstruction, patients also present with epistaxis, which may be significant. Because of the potential for severe blood loss, these lesions should not be biopsied in an uncontrolled setting. These lesions tend to originate at the sphenopalatine foramen and then grow medially into the nasal cavity, laterally into the pterygopalatine fossa, and posteriorly into the nasopharynx (Figure 3A). Imaging is critical for not only diagnosing JAF, but also for precise mapping prior to surgical resection. Catheter angiography with preoperative embolization is often performed to minimize blood loss during surgery. MRI is the modality of choice for JAF evaluation. JAF are isointense on T1 weighted images and hyperintense on T2/STIR sequences compared to other soft tissues. Because of the highly vascular nature of these tumors, avid enhancement is typical on post-gadolinium T1 weighted fat-suppressed images. Flow voids may be seen on both long TR and postcontrast sequences (Figures 3B, 3C).
Tumors of bone and cartilage
A variety of benign neoplasms and fibro-osseous lesions arise from the bones and cartilage of the face and SN cavities. The term “fibro-osseous” generally refers to a category of benign neoplasms or tumor-like lesions, with osteoma, ossifying fibroma, and fibrous dysplasia being the most common. Fibrous dysplasia is a non-neoplastic tumor-like condition and is common in the craniofacial skeleton. As it is non-neoplastic, it will not be discussed further, but should be recognized as a mimic of neoplastic disease.
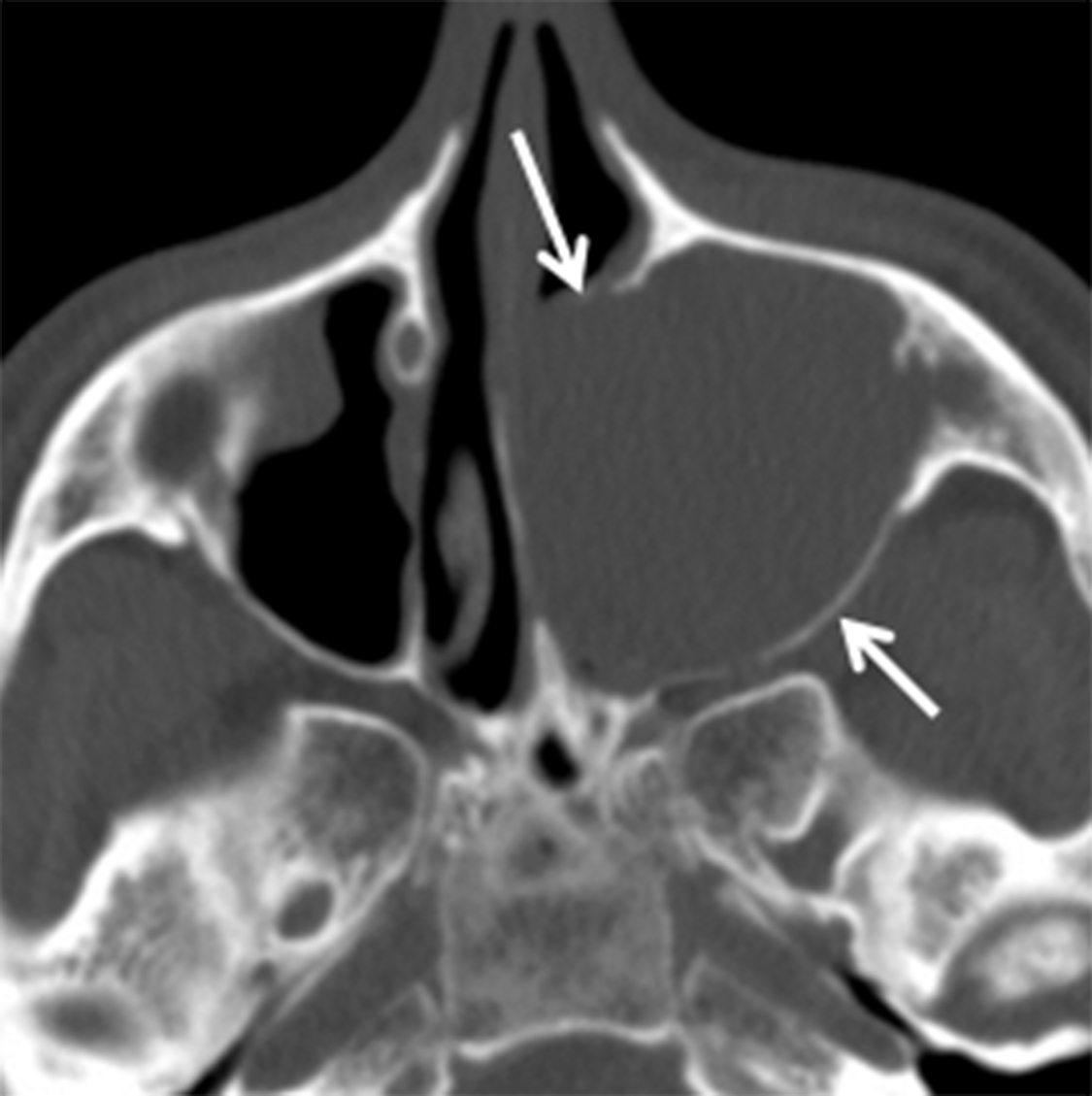
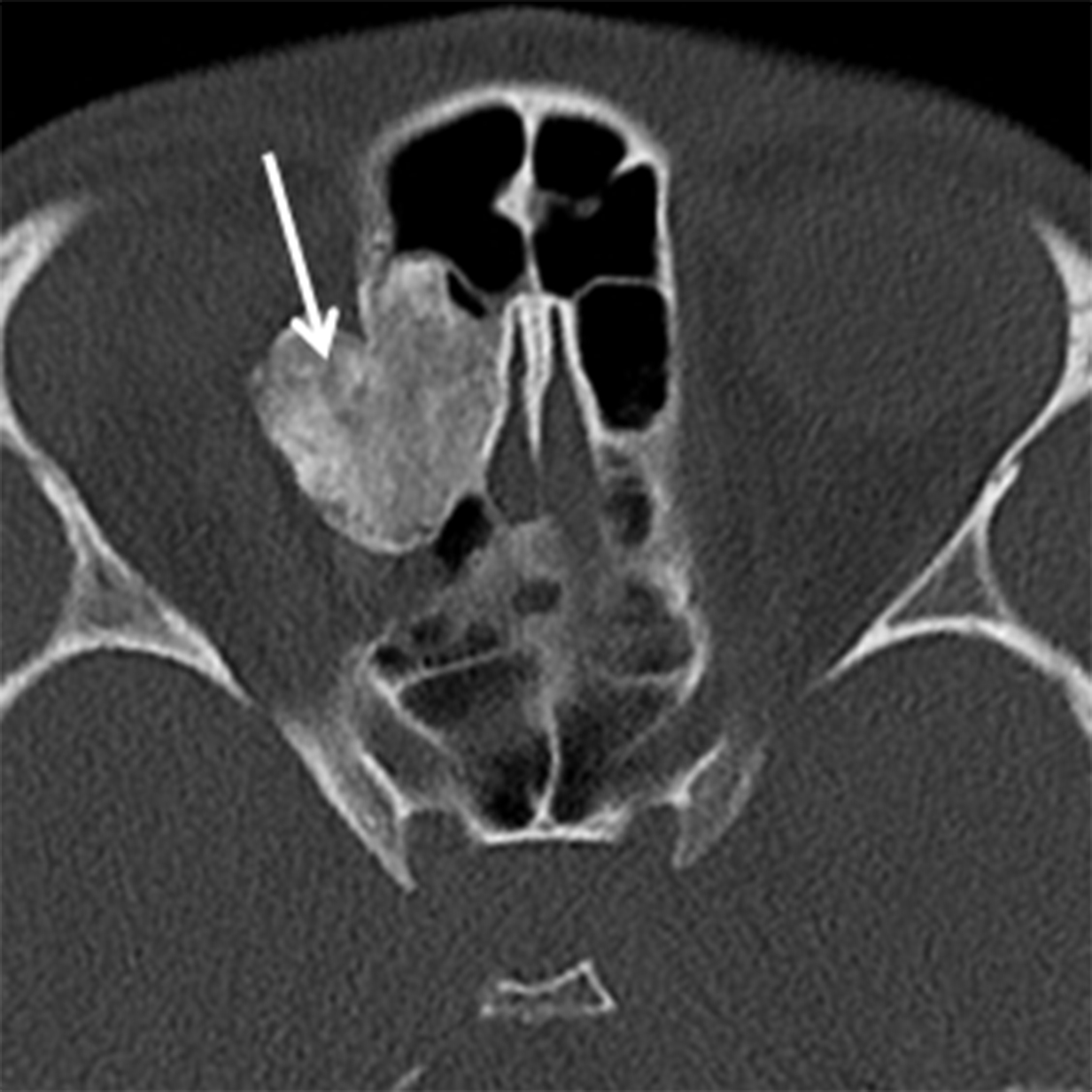
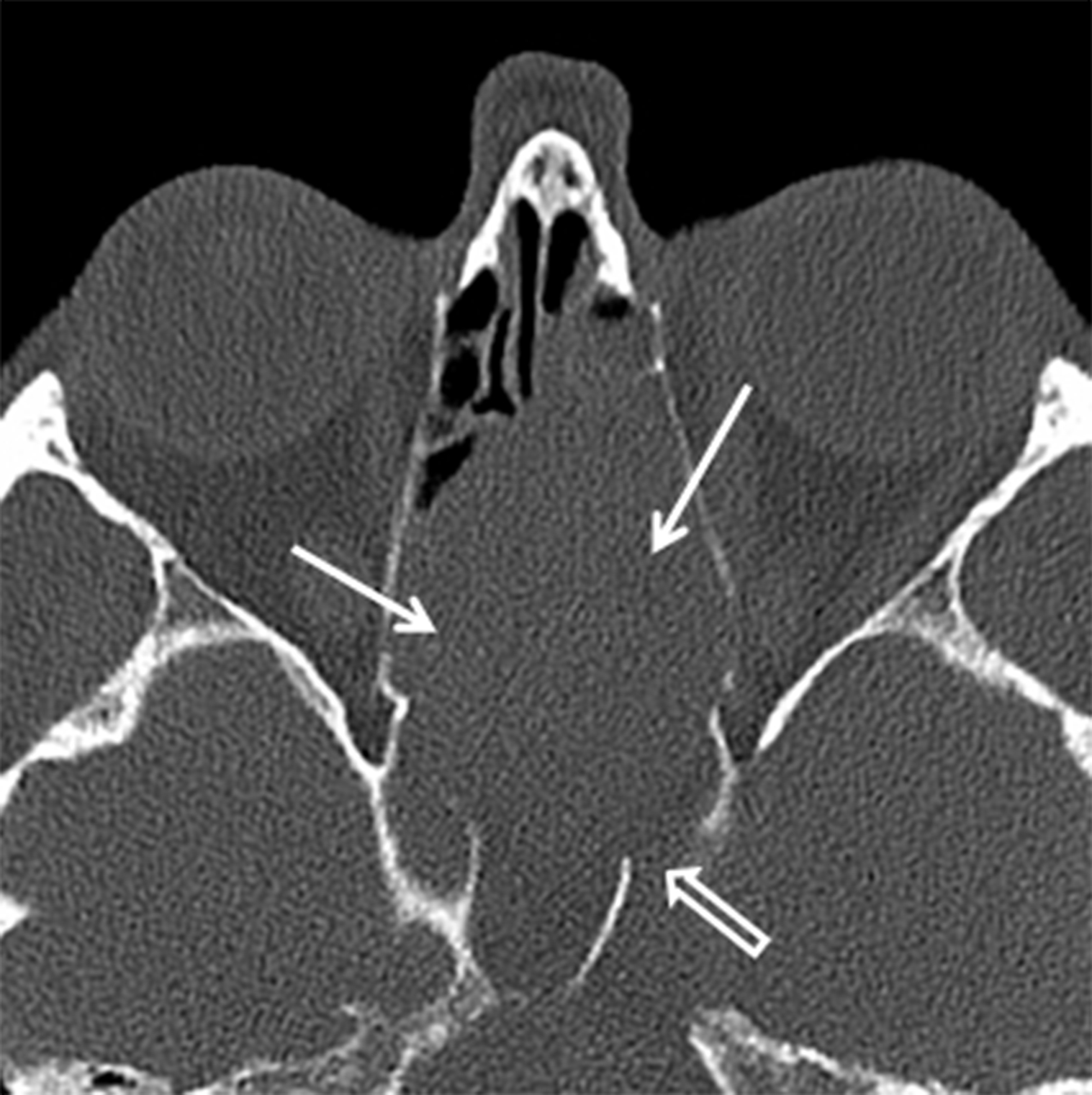
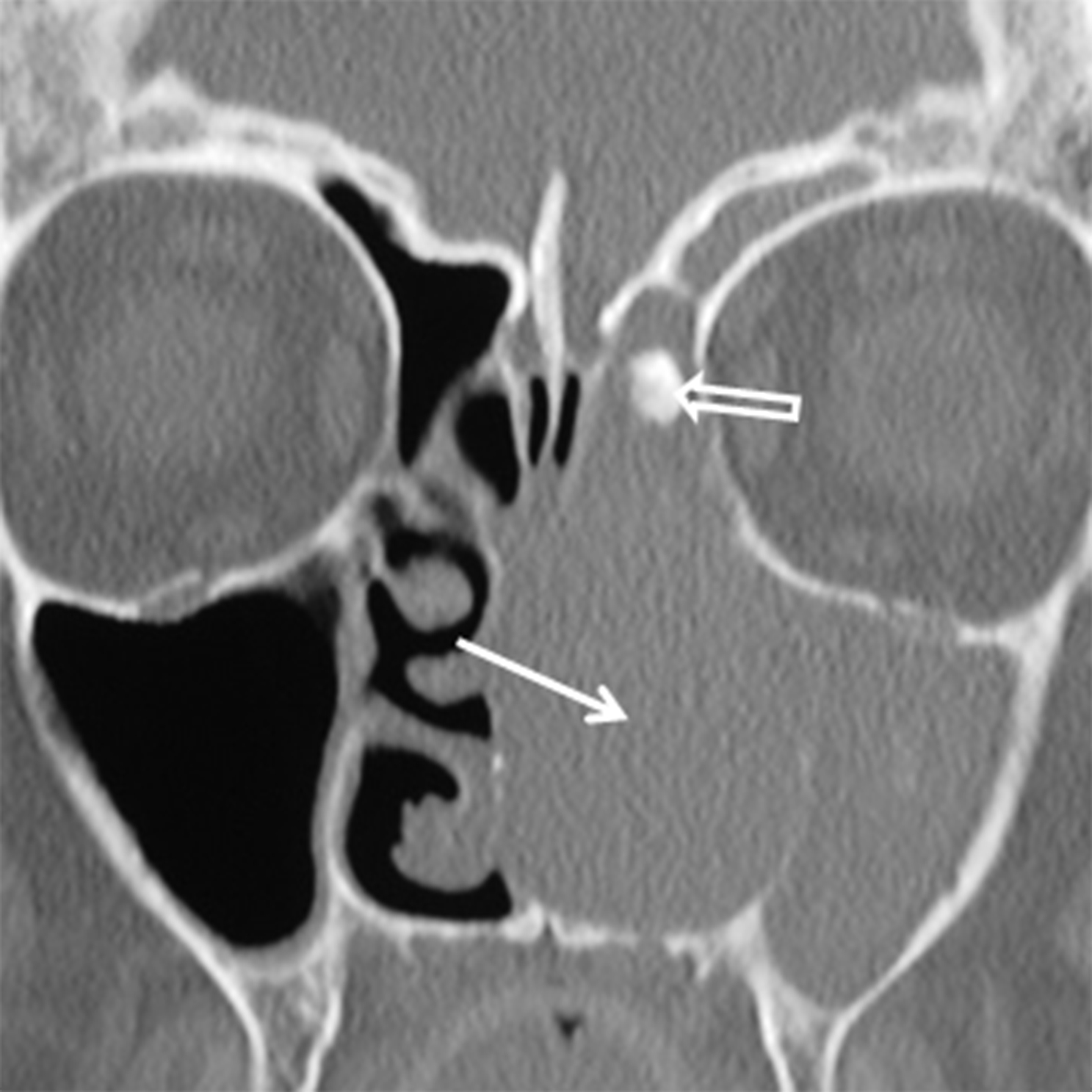
Osteomas are the most common neoplasms of the sinuses and are usually found in the frontal or ethmoid sinuses. The incidence is 1-3% of the population and < 5% are symptomatic.12 Osteomas are more common in men (M:F ≈ 2:1) and are often found incidentally in patients over the age of 50 years.13-14 Multiple osteomas may be seen in the setting of Gardner syndrome. They are best evaluated using thin-slice CT and the classic compact or “ivory” osteoma appears as a dense, ossified lesion attached to a bony sinus wall (Figure 4A). MR is typically not indicated to make the diagnosis, but may be helpful for surgical planning when large osteomas produce orbital or intracranial complications. Osteomas, like all fibro-osseous lesions, demonstrate areas of hypointense signal on T1 and long TR sequences in areas that are ossified or densely fibrous (Figure 4B). Enhancement can be highly variable, occurring in less ossified areas of the tumor.
Ossifying fibromas are lesions composed of mature bone and fibrous tissue thought to arise from mesenchyme of periodontal ligament. They are most often seen in the mandible, but also occur in the maxilla and sinus walls. They are often discovered incidentally, can be locally aggressive, and are more commonly seen in females (F:M 5:1) ages 20-40.15 Imaging characteristics may overlap with fibrous dysplasia and osteoma, and the imaging appearance varies with the amount of fibrous and osseous tissue that is present. Classically, early stage ossifying fibromas have a thick peripheral rim of bone surrounding a soft-tissue density fibrous center.13,16 Late stage lesions show progressive filling of the center of the lesion with mature bone (Figure 5). Similar to osteomas, these lesions are best imaged with CT, with MRI serving as a complementary examination if complications are suspected or surgical planning is required.
Malignant neoplasms
Malignant SN neoplasms represent 3% of head and neck cancers.10 According to the WHO classification, there are 44 different histologic types of SN malignancies and the vast majority are of epithelial origin. While these malignancies tend to be diagnosed at an advanced stage compared to other head and neck cancers, early recognition and correct diagnosis can significantly decrease patient morbidity and improve survival.
Epithelial malignancies
There are 19 epithelial malignancies, including squamous cell carcinoma and its variants, intestinal and non-intestinal-type adenocarcinomas, salivary gland-type carcinomas and neuroendocrine tumors. Squamous cell carcinoma (SCCa) is the most common epithelial malignancy and represents approximately 50-80% of all SN tumors.17 SCCa and adenocarcinoma are more commonly seen in males, SCCa by a 2:1 ratio and adenocarcinoma by up to a 6:1 ratio.17 Both are most common in the 50-60 age range.18 Occupational exposures are a major risk factor for both of these malignancies. Inhaled wood dust, chrome pigment and nickel, to name a few, are considered risk factors and can lead to a 20-fold increased risk of SCCa and 600- to 800-fold increased risk of adenocarcinoma.17 HPV and tobacco smoke are lesser risk factors.18
SCCa and adenocarcinoma can have similar appearances on CT, typically appearing as a solid, heterogeneous mass with areas of necrosis and irregular margins with osseous destruction (Figures 6A,6B). The MR appearances may also be similar with a heterogeneous, typically T2 hyperintense lesion with variable contrast enhancement (Figure 6C). Intrinsic T1 hyperintensity may be seen in areas of necrosis related to blood products. Both lesions show intense FDG uptake on PET imaging.19-20
Although the imaging features of SCCa and adenocarcinoma may overlap, differentiating features do exist and the most helpful is tumor location. SN SCCa most often originates in the maxillary antrum (≈ 60-70%) and the nasal cavity is the second most common site of origin (≈ 12-25%).21 Adenocarcinoma has a predilection for the ethmoid sinuses.20 SCCa also tends to enhance less avidly and have less well-defined margins compared to adenocarcinoma.
Sinonasal undifferentiated carcinoma (SNUC) is an aggressive neoplasm without evidence of squamous or glandular differentiation. Some authors have speculated a neuroendocrine origin as there are some similarities with other neoplasms in that class. SNUC is often treated as a distinct histologic entity. A male predominance is reported (2-3:1) and the tumor occurs in a wide age range with cases reported from the 3rd to 9th decades.22 Nodal involvement and distant metastasis are more commonly seen with SNUC compared to other SN malignancies. SNUC has a very poor prognosis with reported 5-year survival of less than 20%.23 Imaging of SNUC typically reveals a large, destructive mass with ill-defined margins and areas of necrosis (Figure 7).10
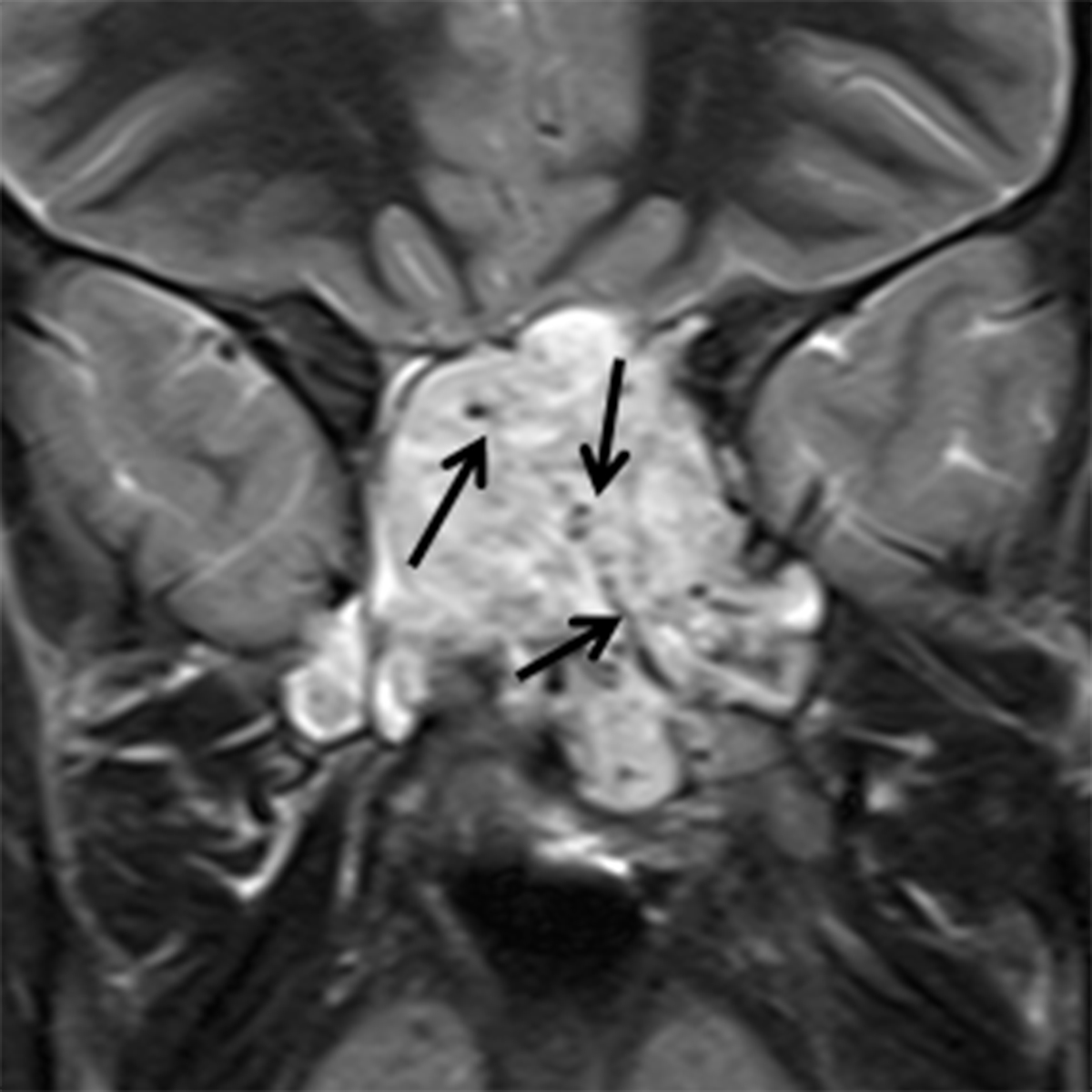
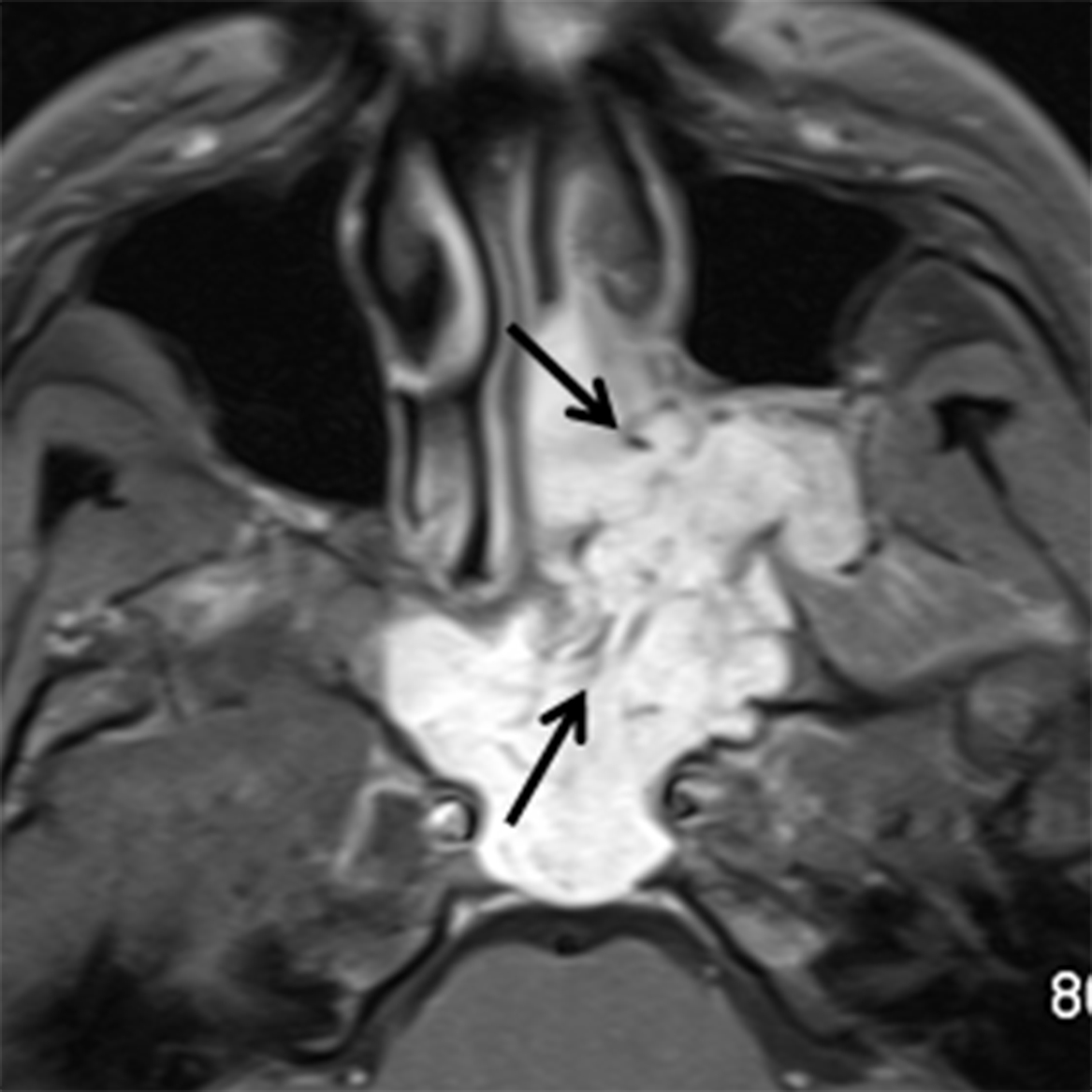
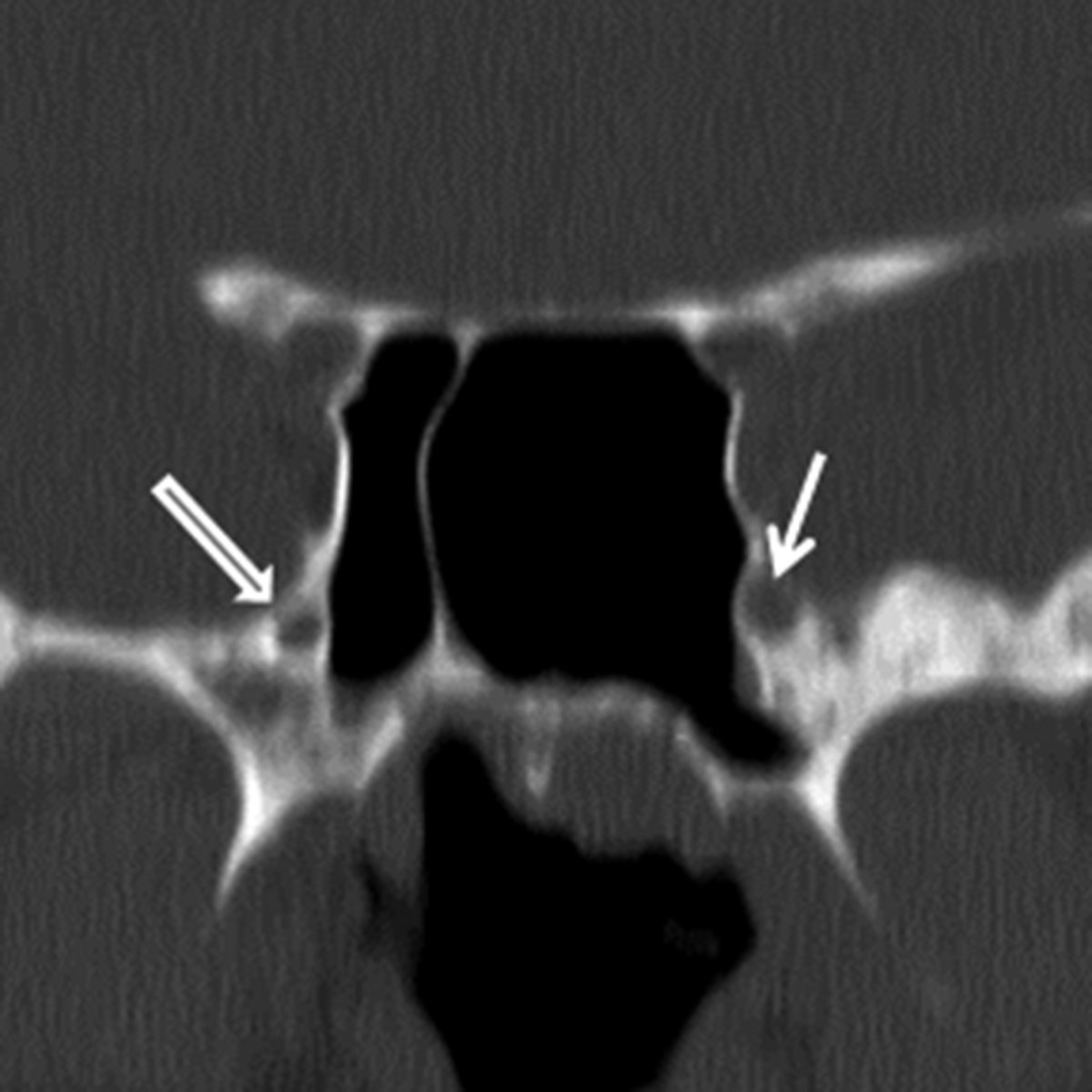
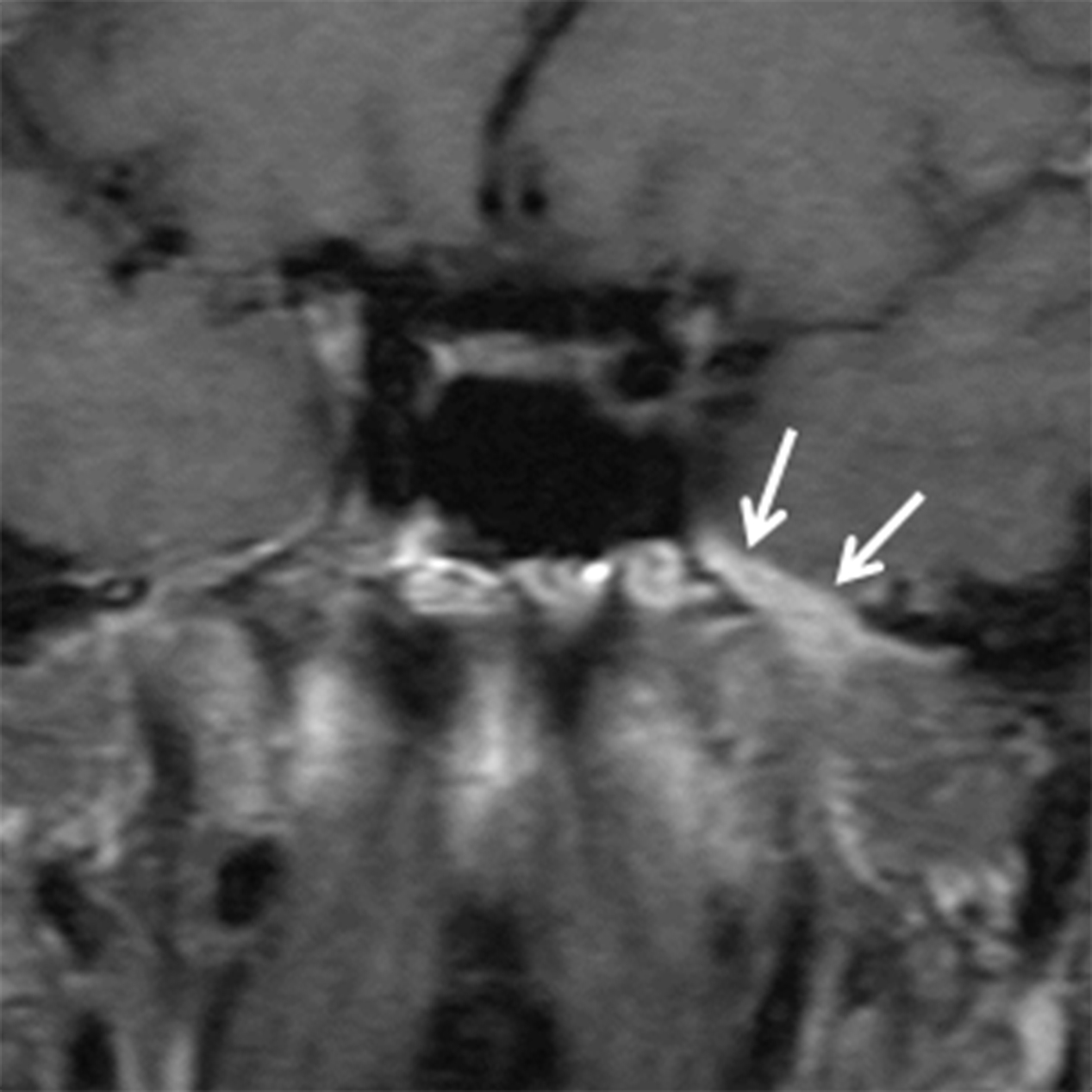
While many types of salivary gland-type malignancies exist, the most common to involve the SN cavities is adenoid cystic carcinoma (ACCa). ACCa is slightly more common in men (1.3:1) and typically occurs in the 40- to 60-year age range.24 The most common site of origin is the maxillary sinus followed by the nasal cavity, ethmoid, and sphenoid sinuses. ACCa has a propensity for perineural spread (PNTS) and subsequent intracranial involvement. The maxillary division of the trigeminal nerve (V2) is most commonly involved given its proximity to the maxillary sinus.10, 20 Detection of PNTS is critical for patient management as its presence may significantly increase surgical morbidity, deem the patient inoperable, or alter the radiation field.25
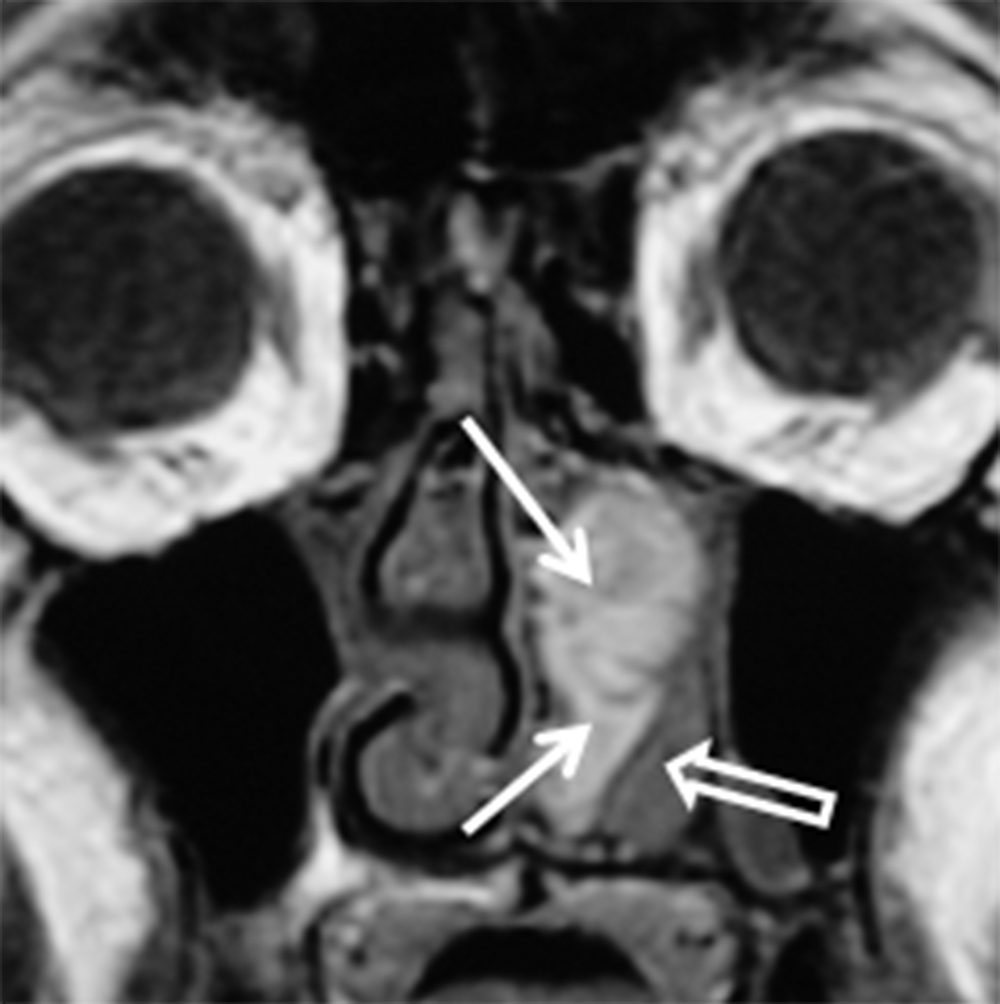
CT typically demonstrates a solid mass in the maxillary sinus or nasal cavity (Figure 8A). Lower grade tumors tend to enhance homogenously and remodel bone, whereas higher grade lesions often demonstrate non-enhancing, necrotic areas and osseous destruction. CT may also show enlargement of bony neural foramina in cases of PNTS. (Figure 8B) MR is the preferred modality for mapping ACCa and detecting PNTS. ACCa typically shows iso- to hypointense signal on T1 and long TR sequences. Enhancement is heterogeneous in higher grade lesions and areas of necrosis may be seen. The involved nerve may be enlarged and shows abnormal increased enhancement compared to the contralateral side (Figure 8C).
Small cell neuroendocrine carcinoma (SNEC) is a rare SN neoplasm with neuroendocrine features. There have been < 100 reported cases in the literature. Sinonasal SNEC is typically seen in the sixth decade with a slight male prevalence.26 No known risk factors are identified.27-28 These tumors typically appear as a large destructive sinonasal mass with a predilection for the ethmoid sinuses (Figure 9). SNEC tends to be extremely aggressive, similar to SNEC in other parts of the body, and has an extremely poor prognosis.
Hematolymphoid neoplasms
Lymphoma, plasmacytoma, myeloid and histiocytic sarcomas, and Langerhans cell histiocytosis are included in this group of lesions. Non-Hodgkin lymphoma (NHL) is the most common non-epithelial neoplasm of the SN cavities and represents approximately 0.2-2% of all NLH.29 The most common type of SN lymphoma in western countries is B-cell NHL. T-cell/natural killer (NK) cell subtypes are more commonly seen in Asia.29 B-cell NHL occurs equally in both genders and typically presents in the sixth decade.29
SN lymphoma has a predilection to originate in the nasal cavity compared to the paranasal sinuses. CT typically reveals a bulky, lobulated mass with osseous remodeling or destruction that is slightly hyperdense on non-contrast CT (Figure 10A). Increased attenuation, if seen, is due to high cellularity and high nuclear to cytoplasmic ratio within the cells.10,30 Variable, but homogeneous enhancement is typical because of the homogeneous cell population. Necrosis is uncommon compared to other malignancies, but is more common in the NK subtype. MRI typically reveals a lesion that is isointense to other soft tissues on T1 with homogenous enhancement. A characteristic feature of NHL is hypointense signal on long TR (T2/STIR) sequences compared to muscle, again due to the high cellularity and N:C ratio 10, 30 (Figure 10B).
Neuroectodermal malignancies
Esthesioneuroblastoma (ENB) is a rare neuroendocrine tumor that arises from the neural cells of the olfactory mucosa. ENB accounts for 3% of SN tumors.31 A slight male predominance has been reported and there are no known risk factors.31 There is a bimodal age distribution for this tumor. The majority of cases occur between 45-55 years of age with a smaller second peak between the ages of 10-25. ENB is a highly vascular tumor and epistaxis may be a presenting symptom. Nodal involvement occurs in 10-15% of cases and recurrences are common, reported in up to 50% of treated cases.32 Despite the presence of orbital and/or intracranial involvement, outcomes are typically better for ENB than other SN malignancies with a 75% 5-year survival. 32
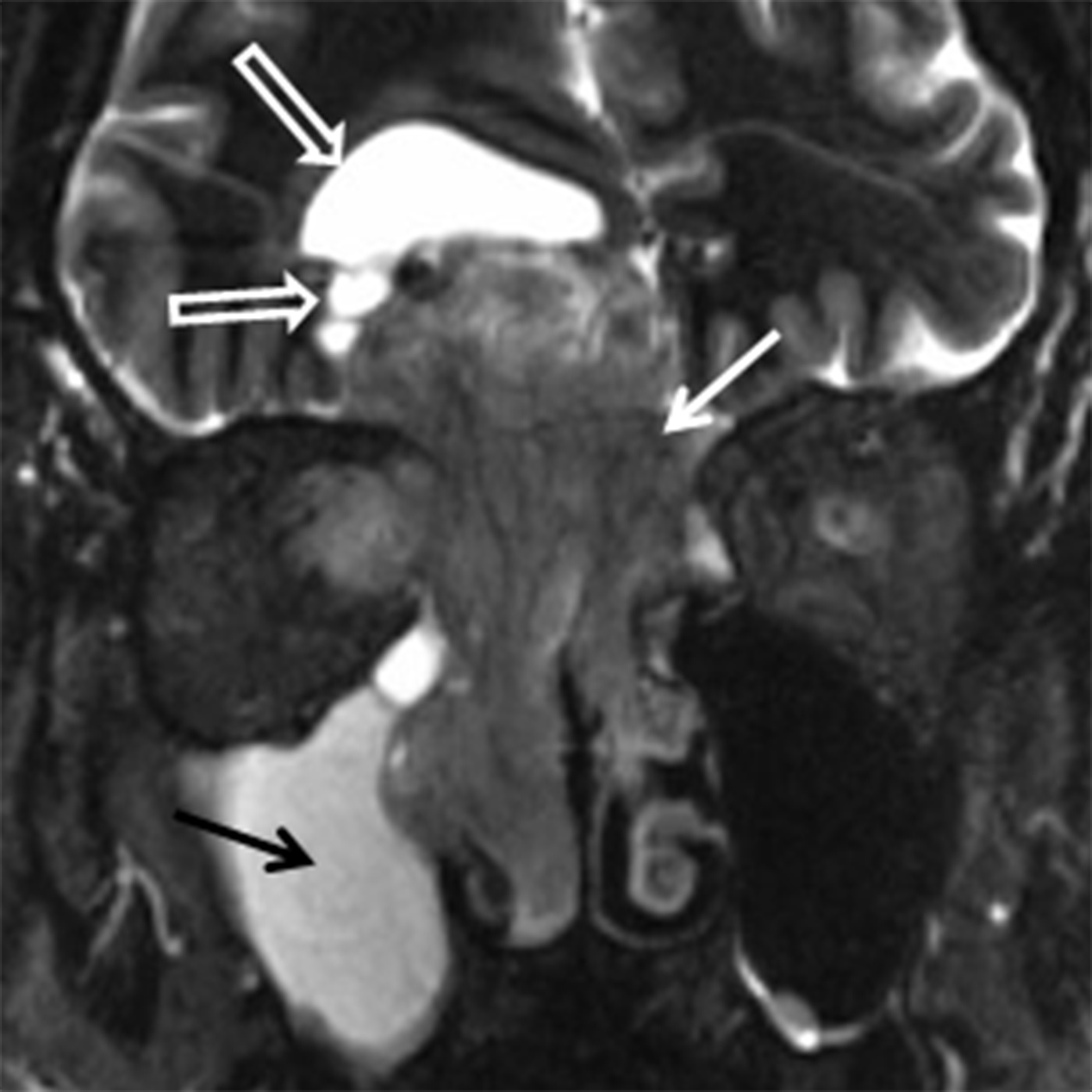
ENB arises in the nasal cavity typically near the cribriform plate, but may occur inferiorly to the level of the middle turbinate. (Figure 11A) These lesions typically show hyperintense long TR signal, enhance avidly, and may demonstrate the presence of flow voids due to the high vascularity (Figure 11B). Intracranial extension is not uncommon and a “waist” may be seen at the anterior skull base, giving the tumor a dumb-bell shape. Lesions that extend intracranially may show cyst formation at the tumor-brain interface which is a distinguishing feature of ENB when present20, 31 (Figure 11C).
Sinonasal melanoma (SNM) is a rare mucosal melanoma, accounting for less than 1% of all melanomas.33 Studies are limited, but suggest that SNM has equal gender prevalence with an average age of occurrence of 64 years. 33 As this lesion is very vascular, patients may present with nasal bleeding. SNM tends to be more aggressive than cutaneous melanomas and has a poor prognosis with a 5-year survival of 5% and a mean survival of 44 months. 33
The CT appearance of SNM can be nonspecific however, this neoplasm has a predilection for the nasal cavity (septum, lateral wall, and inferior turbinate) as opposed to the paranasal sinuses. SNM may remodel or destroy bone. The MR signal characteristics can be suggestive of the diagnosis if the lesion is highly melanotic, with T1 shortening and hypointensity on T2/STIR sequences due to the paramagnetic effect of melanin10, 20 (Figure 12). Variable enhancement is seen, but may be difficult to appreciate in highly melanotic lesions that show T1 shortening.
Malignant tumors of soft tissue, bone and cartilage
Although rare, mesenchymal tumors can occur in the head and neck, arising from muscle, bone, and cartilage. Rhabdomyosarcoma tends to occur in children. Osteosarcoma and chondrosarcoma occur most often adults (fifth - seventh decade).34-35 Survival varies depending on stage and aggressiveness, but overall 5-year survival for mesenchymal tumors is 47%.36 On imaging, lesion matrix of osteosarcoma and chondrosarcoma helps to determine the lesion type. (Figure 13) Chondrosarcoma also demonstrates high signal on long TR images and is usually centered in the midline nasal cavity as it originates from the cartilaginous nasal septum.
Patient management
The TNM classification system is used for carcinomas arising in the maxillary sinus and in the nasal cavity and ethmoid sinuses. There is no TNM staging system for frontal or sphenoid sinus malignancies, although those sites of origin are extremely rare. Some histologies are staged using other systems, for example, the Kadish system is used for staging esthesioneuroblastoma.
The main therapy for benign lesions is complete surgical excision. A description of the various surgical approaches is beyond the scope of this review; however, endoscopic approaches are preferred as they results in less postoperative morbidity.18 Lesions not accessible by endoscopic techniques may be approached using open or combined endoscopic/external approaches.
Malignant lesions are most often treated with a combination of surgical resection and radiation therapy. Chemotherapy may be used preoperatively in an attempt to reduce the size of a large primary and improve resectability or if there is metastatic disease. Effectiveness of therapies is variable as some malignancies show a favorable response to chemotherapy while others are radiosensitive. Long term surveillance is almost always required to monitor for recurrence.37
Conclusion
The goal of this article was to present a relatively inclusive review of the more commonly encountered or important SN neoplasms. It is important for the imager to recognize findings that suggest a neoplasm, as these patients often present with nonspecific symptoms, are suspected to have inflammatory disease, and are initially evaluated with CT. Delays in diagnosis are common and can lead to significant morbidity and increased mortality. Early detection and definitive tissue diagnosis can markedly improve outcomes. Surgical resection is often the primary treatment modality with adjuvant chemotherapy and/or radiotherapy used for malignant neoplasms.
REFERENCES
- Barnes L, Tse LLY, Hunt JL, et al. In: Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and Genetics of Head and Neck tumors. Lyon, FR: IARC Press; 2005:9-80.
- Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26(2):157-163.
- von Buchwald C, Bradley PJ. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007;15(2):95-98.
- Caruana SM, Zwiebel N, Cocker R, et al. p53alteration and human papilloma virus infection in paranasal sinus cancer. Cancer. 1997;79(7):1320-1328.
- Beck JC, McClatchey KD, Lesperance MM, et al. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113(5):558-563.
- Ojiri H, Ujita M, Tada S, et al. Potentially distinctive features of sinonasal inverted papilloma on MR imaging. AJR Am J Roentgenol. 2000;175(2):465-468.
- Oztürk E, Sağlam O, Sönmez G, et al. CT and MRI of an unusual intranasal mass: pleomorphic adenoma. Diagn Interv Radiol. 2008;14(4):186-188.
- Yu E, Mikulis D, Nag S. CT and MR imaging findings in sinonasal schwannoma. AJNR Am J Neuroradiol. 2006;27(4):929-930.
- Cakmak O, Yavuz H, Yucel T. Nasal and paranasal sinus schwannomas. Eur Arch Otorhinolaryngol. 2003;260(4):195-197.
- Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
- Lloyd C, McHugh K. The role of radiology in head and neck tumours in children. Cancer Imaging. 2010;10:49-61.
- Erdogan N, Demir U, Songu M, et al. A prospective study of paranasal sinus osteomas in 1,889 cases: changing patterns of localization. Laryngoscope. 2009;119(12):2355-2359.
- Eller R, Sillers M. Common fibro-osseous lesions of the paranasal sinuses. Otolaryngol Clin North Am. 2006;39(3):585-600
- McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head NeckPathol. 2013 Mar;7:5-10.
- Alawi F. Benign fibro-osseous diseases of the maxillofacial bones. A review and differential diagnosis. Am J Clin Pathol. 2002;118 Suppl:S50-70.
- Engelbrecht V, Preis S, Hassler W, et al. CT and MRI of congenital sinonasal ossifying fibroma. Neuroradiology. 1999;41(7):526-529.
- Llorente JL, López F, Suárez C, et al. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11(8):460-472.
- Haerle SK, Gullane PJ, Witterick IJ, et al. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24(1):39-49.
- Gomaa MA, Hammad MS, Abdelmoghny A, et al. Magnetic resonance imaging versus computed tomography and different imaging modalities in evaluation of sinonasal neoplasms diagnosed by histopathology. Clin Med Insights Ear Nose Throat. 2013;26(6):9-15.
- Das S, Kirsch CF. Imaging of lumps and bumps in the nose: a review of sinonasal tumours. Cancer Imaging. 2005;9(5):167-77.
- Pilch BZ, Bouquot J, Thompson LDR. In: Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and Genetics of Head and Neck Tumors. Lyon, FR: IARC Press; 2005:9-80.
- Wenig BM. Undifferentiated malignant neoplasms of the sinonasal tract. Arch Pathol Lab Med. 2009;133(5):699-712.
- Frierson HF Jr, Mills SE, Fechner RE, et al. Sinonasal undifferentiated carcinoma: an aggressive neoplasm derived from Schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10 (11):771–779.
- Husain Q, Kanumuri VV, Svider PF, et al. Sinonasal adenoid cystic carcinoma: systematic review of survival and treatment strategies. Otolaryngol Head Neck Surg. 2013;148(1):29-39.
- Lupinetti AD, Roberts DB, Williams MD, et al. Sinonasal adenoid cystic carcinoma: the M.D. Anderson Cancer Center experience. Cancer. 2007;110(12):2726-2731.
- Chai L, Ying HF, Wu TT, et al. Clinical features and hypoxic marker expression of primary sinonasal and laryngeal small-cell neuroendocrine carcinoma: a small case series. World J Surg Oncol. 2014 ;12:199.
- Sirsath NT, Babu KG, Das U, Premlatha CS. Paranasal sinus neuroendocrine carcinoma: a case report and review of the literature. Case Rep Oncol Med. 2013;2013:728479. Epub 2013 Feb 14.
- Krishnamurthy A, Ravi P, Vijayalakshmi R, et al. Small cell neuroendocrine carcinoma of the paranasal sinus. Natl J Maxillofac Surg. 2013 ;4(1):111-113.
- Shohat I, Berkowicz M, Dori S, et al. Primary non-Hodgkin’s lymphoma of the sinonasal tract. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(3):328-331.
- Neves MC, Lessa MM, Voegels RL, et al. Primary non-Hodgkin’s lymphoma of the frontal sinus: case report and review of the literature. Ear Nose Throat J. 2005;84(1):47-51.
- Ow TJ, Bell D, Kupferman ME, et al. Esthesioneuroblastoma. Neurosurg Clin N Am. 2013;24:51-65.
- Bak M, Wein RO. Esthesioneuroblastoma: a contemporary review of diagnosis and management. Hematol Oncol Clin North Am. 2012;26(6):1185-1207.
- Clifton N, Harrison L, Bradley PJ, et al. Malignant melanoma of nasal cavity and paranasal sinuses: report of 24 patients and literature review. Laryngol Otol. 2011;125(5):479-485.
- Palacios E, Quiroz-Casian A, Garza Garcia L, et al. Adult case of large sinonasal embryonal rhabdomyosarcoma with intracranial extension. Ear Nose Throat J. 2013;92(4-5):177-178.
- Shahid H, Baharudin A, Halim AS, et al. An extensive sinonasal osteosarcoma mimicking chondrosarcoma. Med J Malaysia. 2007;62(2):171-172.
- Wu AW, Suh JD, Metson R, et al. Prognostic factors in sinonasal sarcomas: analysis of the surveillance, epidemiology and end result database. Laryngoscope. 2012;122(10):2137-2142.
- Ansa B, Goodman M, Ward K, et al. Paranasal sinus squamous cell carcinoma incidence and survival based on surveillance. Epidemiology, and end results data, 1973 to 2009. Cancer. 2013;119(14):2602-2610.
Citation
KB M, BD H, MA M. Sinonasal neoplasms: Update on classification, imaging features, and management. Appl Radiol. 2015;(12):7-15.
December 17, 2015