See It, Treat It: How Theranostics Is Reshaping Cancer Care and Nuclear Medicine
Theranostics, the integration of diagnostic imaging and targeted therapy, is revolutionizing the field of nuclear medicine. This review explores its origins, clinical applications, future directions, and its potential as a transformative career path for radiologists and nuclear medicine physicians. Beginning with the pioneering use of radioactive iodine, theranostics has evolved into a precision medicine tool with landmark examples like lutetium Lu 177 dotatate (Lutathera) for neuroendocrine tumors and lutetium Lu 177 vipivotide tetraxetan (Pluvicto) for prostate cancer. The concept of ‘theranostic twins’—radioactive drugs sharing a molecular target but used for imaging or therapy—has allowed for unparalleled treatment specificity. We discuss mechanisms of action, clinical trials such as NETTER-1 and PSMAfore, and the emerging role of dosimetry in individualizing treatment. Barriers including regulatory complexity, reimbursement, and infrastructure requirements are analyzed, along with future advances in α emitters, novel ligands, and applications beyond oncology. With growing interest from trainees, evolving training pathways, and increasing institutional investment, theranostics represents a powerful new frontier in personalized medicine.
Keywords: theranostics, radiology, nuclear medicine, molecular imaging, radiopharmaceutical therapy, lutetium-177, Pluvicto, targeted therapy, PET/CT, dosimetry
Introduction
The 1966 science fiction film “Fantastic Voyage” depicted a miniaturized medical team navigating the bloodstream to diagnose and treat a brain lesion. Now, nearly 60 years later, the emerging medical specialty of “theranostics” is realizing this vision in a novel and precise form. Theranostics used diagnostic imaging and molecularly targeted radionuclides thousands of times smaller than the fictional Proteus submarine to diagnose and treat specific cancers. The rapid growth of theranostics in the United States is driving a transformative shift in the field of nuclear medicine.
As theranostics expands, a foundational understanding of theranostic principles and applications is becoming essential for today’s radiologists and nuclear medicine physicians. The term “theranostics,” a portmanteau of “therapy” and “diagnostics” originally coined in 1998, also represents a new career pathway for trainees who are interested in a specialty that combines direct patient care with diagnostic and interventional molecular imaging.
Drs. Saul Hertz and Arthur Roberts at Massachusetts General administered the first theranostic treatment using iodine-131 (¹³¹I) to both image and treat hyperthyroidism on March 31, 1941 (now recognized as Theranostics Day), and subsequently, the first thyroid cancer patient the following year. The use of iodine-123 (¹²³I) for imaging and ¹³¹I for therapy marked the first theranostic pair. The “renaissance” in theranostics has been driven by a combination of progress in understanding cancer biology, the identification of specific molecular targets on cancer cells, and advances in radiochemistry and targeting ligands such as peptides and small molecules. Recent breakthroughs in molecular targeting and radiochemistry have led to the development of highly effective radiopharmaceutical therapies, notably lutetium Lu 177 dotatate (Lutathera) approved by the United States Food and Drug Administration (FDA) for neuroendocrine tumors in 2018, and lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved for prostate cancer in 2022.
The phrase “theranostic pair” refers to 2 radioactive drugs that target the same molecular structure—such as a receptor, transporter, or antigen—that may be expressed on cancer cells, within the tumor microenvironment, or on supporting stromal or immune cells, but incorporate different radionuclides for imaging versus therapy. One isotope is chosen for its imaging properties, and the other is selected for its therapeutic properties (delivering a dose of radiation to kill the targeted cells) ( Figure 1 ).
Conceptual drawing of a radiopharmaceutical made up of a radioactive isotope, linker, and targeting molecule complex which attaches to a target protein on a cancer cell.
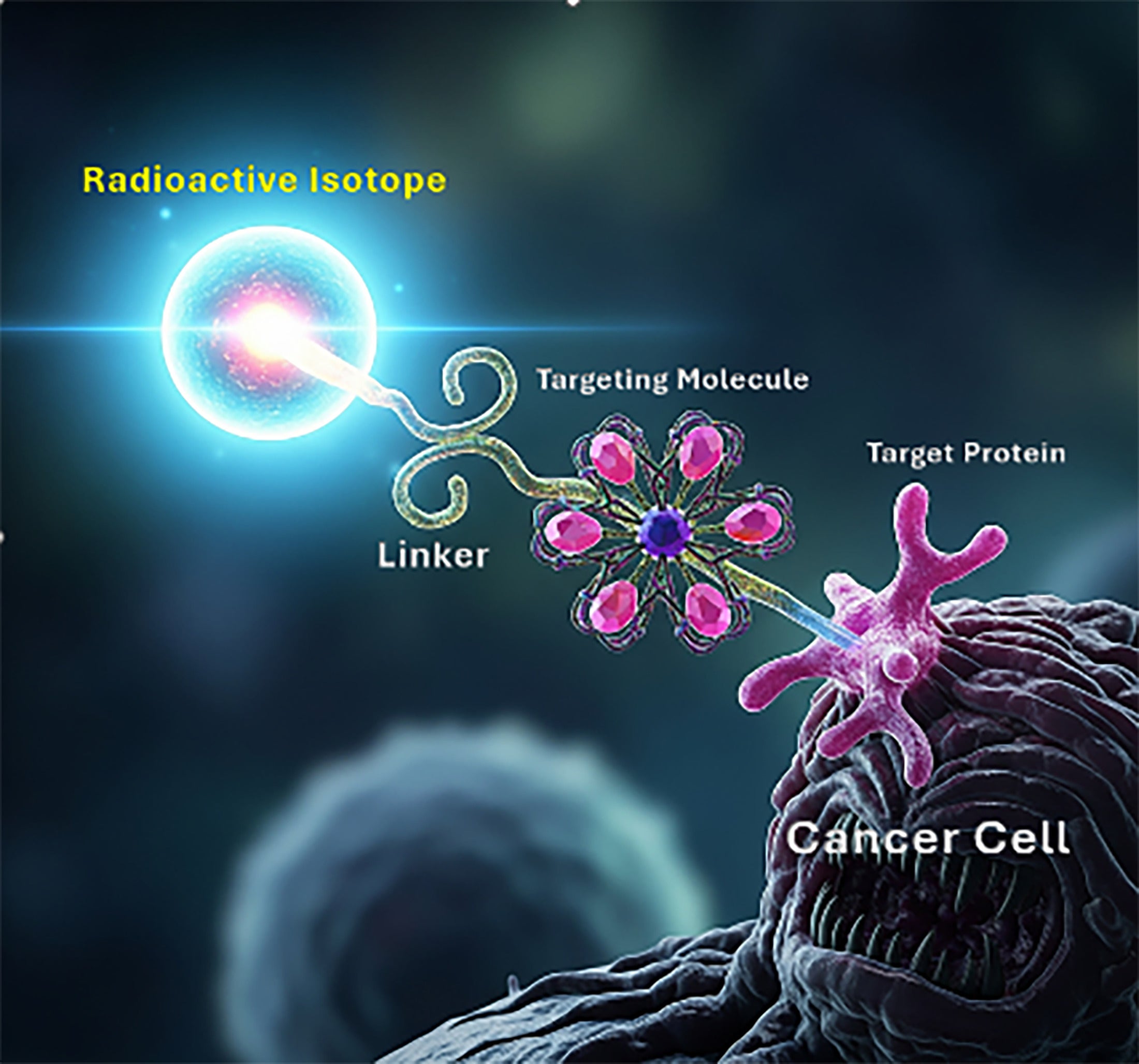
The primary mechanism by which a theranostic molecule attaches to a cancer cell involves a highly selective binding interaction between a targeting ligand on the theranostic molecule and a corresponding receptor or antigen that is either overexpressed or uniquely present on the cancer cell membrane. Common targeting ligands used in theranostic molecules include antibodies, peptides, small molecules, and aptamers ( Table 1 ).
Common Targeting Ligands Used in Theranostic Molecules
| Targeting Ligand Type | Description |
|---|---|
| Antibodies | Proteins that naturally bind with high specificity to certain antigens. Monoclonal antibodies can be engineered to recognize antigens that are abundant on cancer cells. |
| Peptides | Short chains of amino acids that can be designed to bind to specific receptors on cancer cells. |
| Small molecules | Smaller chemical compounds that can be synthesized to target and bind to specific molecules or pathways involved in cancer cell growth and survival. |
| Aptamers | Nucleic acid (DNA or RNA) molecules that can fold into specific 3D structures to bind to target molecules with high affinity and specificity. |
A PET/CT scan is performed using a diagnostic twin such as ⁶⁸Ga-DOTATATE (Gallium-68 DOTA-[Tyr³]-octreotate) (68-minute half-life) or ⁶⁴Cu-DOTATATE (Copper-64 DOTA-[Tyr³]-octreotate) (12.7-hour half-life) to image somatostatin receptors, which are overexpressed in several neuroendocrine tumor types. If the scan shows sufficient uptake of the diagnostic agent, it suggests that the patient is likely to benefit from the therapeutic twin, which in the case of neuroendocrine tumors is lutetium Lu 177 dotatate. Using the same targeting molecule, it travels to and binds to the same cancer cells identified in the scan, then emits a β particle (a high-energy electron) that disrupts the tumor DNA while minimizing radiation exposure to surrounding healthy tissues. Studies such as NETTER-1 provide compelling evidence indicating that patients experience prolonged survival without disease progression and report enhanced quality of life in patients with advanced, progressive, and well-differentiated somatostatin receptor-positive midgut neuroendocrine tumors.1 Progression-free survival (PFS) in patients treated with lutetium Lu 177 dotatate plus Octreotide LAR (long-acting release) improved to 28-29 months compared with 8.4 months with high-dose octreotide LAR alone. The NETTER-2 trial was the first to evaluate lutetium Lu 177 dotatate as a first-line treatment and included patients with grade 2 and grade 3 advanced gastroenteropancreatic neuroendocrine tumors, again comparing long-acting octreotide alone with combination therapy with lutetium Lu 177 dotatate. Median PFS was extended to 22.8 months compared with 8.5 months for patients receiving combination therapy with a higher objective response rate of 43% versus 9% for the combination therapy, with a small percentage of patients in the lutetium Lu 177 dotatate arm achieving a complete response. Patients receiving lutetium Lu 177 dotatate typically undergo 4 cycles of therapy that are 8 weeks apart.
In the case of prostate cancer, the diagnostic twins currently FDA approved are68 Ga-PSMA-11 (gallium-68-labeled prostate-specific membrane antigen-11),18 F-DCFPyL (fluori ne- 1 8 -labeled 2-(3-{1-carboxy-5-[(6-[¹⁸F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid), and18 F-rhPSMA-7.3 (fluorine-18-labeled radiohybrid prostate-specific membrane antigen ligand 7.3). These bind to prostate-specific membrane antigen (PSMA), which is commonly overexpressed in patients with prostate cancer. This enables direct visualization of the PSMA distribution throughout the body. Lutetium Lu 177 vipivotide tetraxetan serves as the therapeutic twin by first binding to the PSMA receptor on the surface of a prostate cancer cell and causing endocytosis of the PSMA receptor-177 Lu-vipivotide tetraxetan complex. Once inside, β particles are released, damaging the cancer cell’s DNA and ultimately leading to cell death ( Figure 2 ).
Mechanism of action for lutetium Lu 177 vipivotide tetraxetan (Pluvicto). The radioligand targets PSMA receptors on prostate cancer cells, is internalized through endocytosis, and releases β particles intracellularly, inducing DNA damage and cell death. Each prostate cancer cell can ingest (internalize) multiple radioligands.
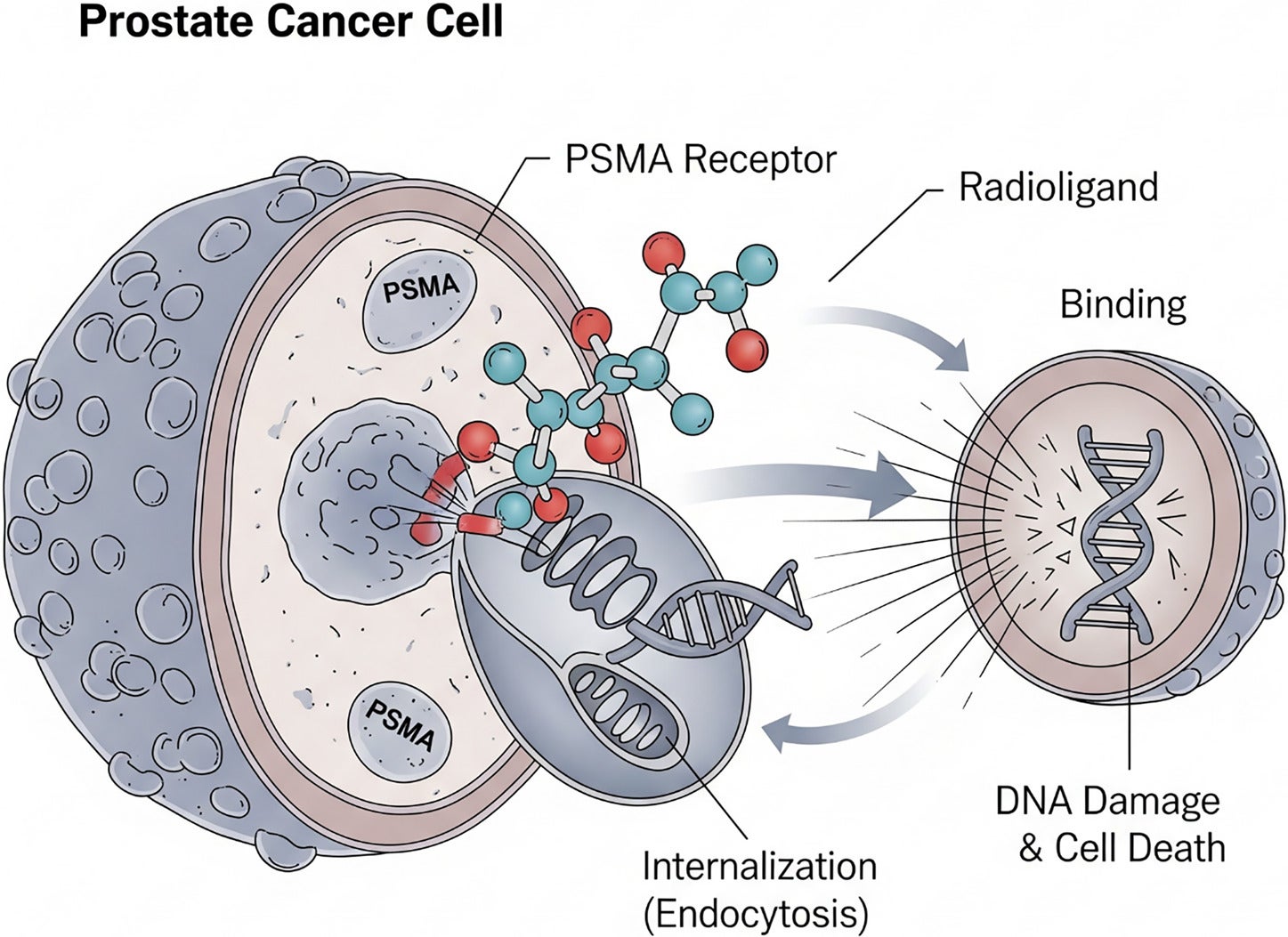
Lutetium Lu 177 vipivotide tetraxetan is currently FDA approved for PSMA-positive metastatic castration-resistant prostate cancer patients who have been treated with an androgen receptor pathway inhibitor and either
-
have previously received taxane-based chemotherapy or
-
as of April 2025 are eligible for treatment prior to receiving taxane-based chemotherapy.
This latest expanded indication for lutetium Lu 177 vipivotide tetraxetan1 was based on the PSMAfore trial. The trial demonstrated that in patients who had progressed after an androgen receptor pathway inhibitor and had not yet received chemotherapy, lutetium Lu 177 vipivotide tetraxetan achieved a 57% reduced risk of radiographic progression or death and more than doubled median radiographic PFS from 5.6 months to 12.0 months compared with patients switched to another androgen receptor pathway inhibitor.2 Additionally, patients treated with lutetium Lu 177 vipivotide tetraxetan had a 51% versus 15% objective response rate (tumor reduction in size demonstrated with imaging) and 58% versus 20% significant decline in PSA levels. Patients receiving lutetium Lu 177 vipivotide tetraxetan typically undergo up to 6 treatment cycles, each spaced 6 weeks apart.
Dosimetry for Theranostics
Unlike chemotherapy, which delivers cytotoxic agents systemically with limited ability to measure in vivo distribution, post-therapy “dosimetry” using SPECT/CT allows precise assessment of how much of the injected dose is delivered to tumor cells as well as organs at risk, such as the kidneys, liver, and bone marrow. Dosimetry data for lutetium Lu 177 dotatate and lutetium Lu 177 vipivotide tetraxetan are obtained through multiple SPECT/CT studies post-therapy. Given the 6.7-day half-life of177 Lu, these studies can track the clearance of the injected radiopharmaceutical from tumors and organs at risk at early (a few hours after injection) and multiple intermediate time points, days after the initial therapy. Given the current “one-dose-fits-all” FDA approval, dosimetry can inform theranostic providers about potential toxicities for organs at risk (eg, renal, liver, and bone marrow), educate patients about their treatment, and provide quality assurance. In the future, when doses and timing of delivery of doses will be customized to individual patients, dosimetry will play a major role in optimizing delivery of the correct dose to tumors while keeping toxicity at acceptable levels. This will enable the optimization of dosing and time tailored to individual patients, iteratively balancing tumor control and organ toxicity in a way that is not possible with other cancer treatments.
What’s Next?
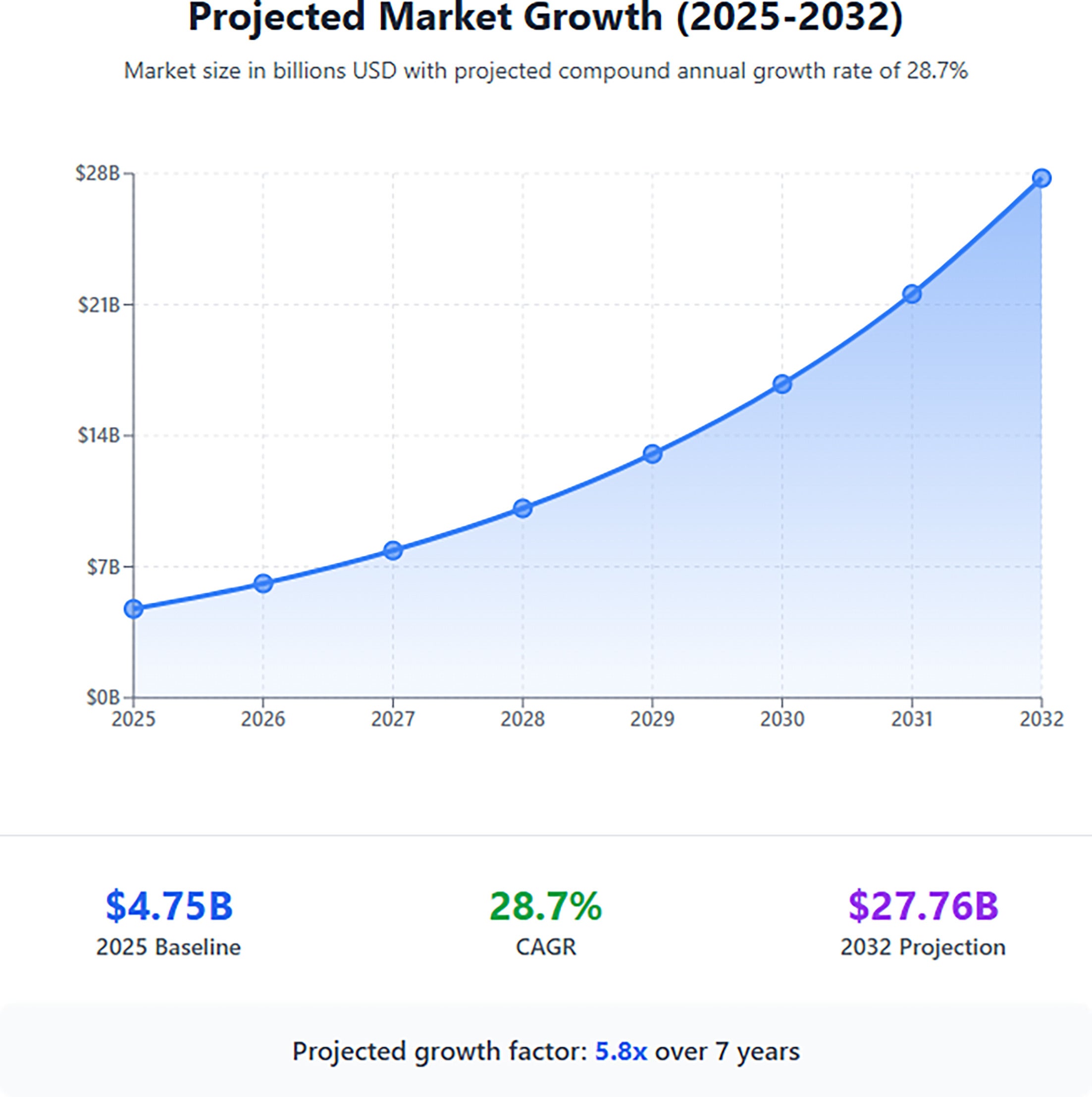
The field of theranostics is advancing at a breathtaking rate, with nanoparticle therapy emerging as a versatile platform for delivering therapeutic agents. The global theranostics market size is also expanding exponentially, driven by significant investments in new radiopharmaceuticals ( Figure 3 ).
Projected growth of the global theranostics market. According to Fortune Business Insights, the market is expected to expand from $4.75 billion in 2025 to $27.76 billion by 2032, representing a compound annual growth rate (CAGR) of 28.7%.
While β emitters such as177 Lu are now well established, α emitters such as actinium-225 are gaining significant traction. α particles are made of a helium nucleus, which is made up of 2 neutrons and 2 protons. α particles have a molecular mass of more than 7000 times that of a β particle and are much more disruptive to both DNA strands of a cancer cell and have a shorter range (50-100 microns). The higher linear energy transfer of α particles allows them to behave more like a scalpel than β particles, which are more akin to a shotgun. By damaging DNA directly and rapidly, their efficacy is unaffected by cell cycle stage, tumor hypoxia, or resistive tumor cell adaptations, as commonly seen with chemotherapy and other types of therapy. Innovative targeting ligands are being engineered to target a much broader range of cancer-specific markers. The 5 cancer types that seem to be on the relatively short-term horizon for theranostic therapy include pancreatic, breast, lung, melanoma, multiple myeloma, and leukemia ( Table 2 ).
The Five Cancer Types on the Near Horizon for Theranostic Therapy and Their Targets
| Cancer Type | Target | Details |
|---|---|---|
| Pancreatic | CLDN4, fibroblast activation protein | Claudins are tight junction proteins that are overexpressed in pancreatic cancer; fibroblasts are abundant in pancreatic and many other types of cancer |
| Breast | HER2, Trop-2 | Well established as targets for antibody-drug conjugates |
| Non-small cell and small cell lung cancer | DLL3, EGFR | DLL3 is an emerging target for small cell lung cancer and other high-grade neuroendocrine tumors; EGFR is a cornerstone target in NSCLC |
| Melanoma | Melanocortin 1 receptor | MC1R, which plays a role in pigmentation, is overexpressed in melanoma cells |
| Multiple myeloma and leukemia | C-X-C chemokine receptor 4 | CXCR4 is involved in how hematopoietic cancer cells traffic to and anchor in the bone marrow |
The field of theranostics is rapidly extending its applications beyond oncology. Researchers are exploring potential applications in cardiovascular medicine, where PET agents can identify molecular markers of inflammation and plaque instability, laying the groundwork for a future theranostic approach.3 Theranostic agents are being designed to visualize and target β-amyloid plaques and tau tangles in Alzheimer’s disease.4 Other potential applications under development include the targeted delivery of antibiotics or antiviral medications to sites of infection and the diagnosis and treatment of autoimmune diseases.
Challenges to the Field of Theranostics
The FDA requires a particularly rigorous and lengthy approval process for combination products (eg, diagnostic and therapeutic twins), which often require separate or coordinated submissions and reviews by multiple FDA centers. Designing clinical trials in humans to effectively prove both safety and efficacy is a complex and highly expensive process. Securing consistent and adequate reimbursement from the Centers for Medicare & Medicaid Services (CMS) and private payors is another key but arduous process, even after FDA approval. While CMS has recently moved to “unbundle” reimbursement for higher-cost diagnostic radiopharmaceuticals, the complexities of billing and coding for both diagnostic and therapeutic components of theranostics remain a significant challenge for health care providers. Supply chain challenges were evident in recent months, including shortages in the global supply of177 Lu and68 Ga, and ramping up domestic production requires major investments in infrastructure such as cyclotrons and processing facilities.
Additionally, the administration of radiopharmaceuticals requires specialized infrastructure, including dedicated shielded infusion rooms, hot labs, PET/CT, and SPECT/CT. The specialized training required to operate the equipment poses recurrent staffing costs from nuclear technicians, specialty-trained radiologists, and nuclear medicine physicians. The sub-specialized knowledge of how to administer these radiopharmaceuticals to patients safely, interpretation of complex multi-modality imaging studies, and the nuanced subtleties and complexities of radiation dosimetry are beyond the experience and training of the vast majority of current inpatient and outpatient diagnostic radiology, radiation oncology, and nuclear medicine practices.
An increasing number of medical students and residents are expressing an interest in a career in this exciting and pioneering field. Theranostics uniquely combines diagnostic imaging with direct patient care guided by a particularly individualized treatment paradigm. Entrepreneurial skills are also necessary to build and grow a thriving practice, as even experienced oncologists and urologists are often unaware of theranostics. As we are seeing at our own institution, an increasing number of applicants are being drawn to nuclear medicine and radiology with the intention of combining their intersecting interests in diagnostic imaging, oncology, and patient care to establish a theranostics practice.
Theranostics training is being incorporated into nuclear medicine residency programs as a foundational part of future practice, as well as part of the American Board of Radiology (ABR) 16-month pathway within a diagnostic radiology residency that can lead to a sub-specialty certificate in nuclear radiology. They are also being incorporated into radiation oncology training programs. Dedicated formal fellowship programs are currently being offered at several academic medical centers, including the University of Washington, Massachusetts General Hospital, Duke University, the University of Iowa, and Wake Forest University. These typically offer a special focus on oncologic nuclear medicine and radiopharmaceutical therapies.
In the future, as theranostics becomes increasingly widespread for a growing diversity of cancers, one could imagine an additional track in diagnostic radiology training programs in addition to the internship plus 4-year diagnostic radiology and 6-year interventional radiology (that includes the internship year) pathways that would focus on “interventional targeted molecular therapy” with added experience in direct patient care, similar to that offered in the interventional radiology with an emphasis on ownership of the patient’s oncologic journey through long-term follow-up and individualized treatment regimens.
Conclusion
The rapidly developing field of theranostics represents a paradigm shift in precision medicine, evolving from a conceptual “fantastic voyage” to a clinical reality that will reshape the landscape of diagnostic radiology, nuclear medicine, and oncology. While significant challenges in regulatory approval, reimbursement, and infrastructure remain, the rapid pace of innovation, from novel targeting ligands and α emitters to expanding applications in cardiovascular and neurological disease, promises an even more impactful future. For radiology and nuclear medicine residents, this dynamic field presents an exciting and rewarding career pathway, merging the intellectual rigor of diagnostic interpretation with the profound fulfillment of direct, life-altering patient therapy. Theranostics is poised to become a new cornerstone of 21st century medicine, offering renewed hope for patients and a new frontier for the next generation of physicians.
References
Citation
Amindarolzarbi A, Siegel E. See It, Treat It: How Theranostics Is Reshaping Cancer Care and Nuclear Medicine. Appl Radiol. 2025;(3):5 - 10.
doi:10.37549/AR-D-25-0084
July 1, 2025