Retroperitoneal Leiomyosarcoma Invading the Inferior Vena Cava
Case Summary
An adult with a history of type 2 diabetes and hypertension presented with abdominal pain. Physical examination demonstrated abdominal fullness. Complete blood count and basic metabolic panel were within normal limits with preserved renal function. Imaging demonstrated a large retroperitoneal mass with invasion into the inferior vena cava (IVC). The tumor was unresectable due to extensive vascular involvement. CT-guided core biopsy was performed. The patient subsequently developed a pulmonary embolism, with US imaging negative for deep vein thrombosis (DVT). Given the near-total occlusion of the IVC by tumor thrombus and lack of other explanatory mechanisms (eg, DVT), the embolism was presumed to be secondary to tumor thrombus. The patient was started on apixaban with and symptoms resolved.
Imaging Findings
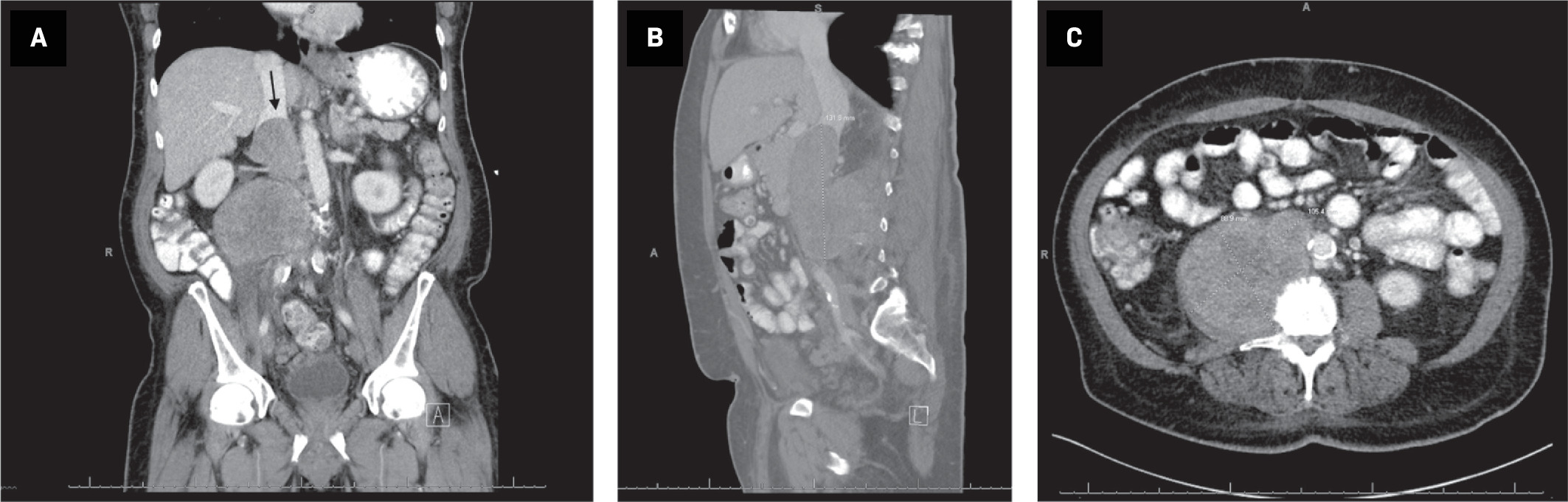
CT of the abdomen and pelvis with oral and intravenous contrast demonstrated a 10.5 × 8.9 × 13.2-cm mass extending from the right psoas muscle into the IVC, indicating a retroperitoneal tumor with IVC extension ( Figure 1 ). Tumor invaded the IVC at the level of the renal veins, with extension superior to the intrahepatic IVC ( Figure 2 ). CT-guided core biopsy demonstrated immunohistochemical staining consistent with leiomyosarcoma.
Coronal reformation of CT of (A) the abdomen/pelvis with oral and intravenous contrast demonstrates a retroperitoneal mass extending into the intrahepatic IVC, (arrow). Sagittal reformation (B) demonstrates IVC invasion by the tumor. Axial CT (C) demonstrates the large, heterogeneous mass originating from the right psoas muscle.
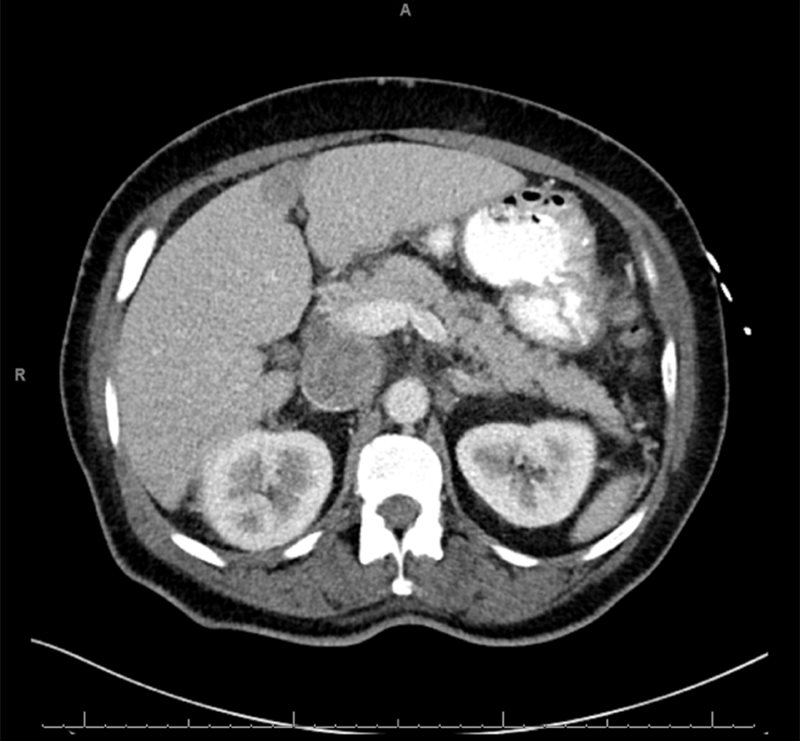
Contrast-enhanced imaging demonstrates obstruction of the suprarenal IVC.
Diagnosis
Leiomyosarcoma.
The differential diagnosis of retroperitoneal masses includes liposarcoma; lymphadenopathy or lymphoma, neurofibroma; and nonmalignant pathologies, including hematoma.
Discussion
Leiomyosarcoma is a malignant tumor demonstrating smooth muscle differentiation occurring in the retroperitoneum in 10-20% of cases.1 It is the second most common sarcoma in the retroperitoneum after liposarcoma.2 Poorly differentiated liposarcomas contain variable amounts of fat, making it more challenging to distinguish these from leiomyosarcoma.2 When leiomyosarcomas involve the IVC, they account for approximately 0.5% of adult soft-tissue sarcomas.3 Retroperitoneal masses often grow to large sizes before detection and are often detected incidentally during imaging for other indications or secondary to compressive symptoms.2 Contributing to this delayed picture is the relative sparing of visceral and vascular structures; however, those affecting the vasculature often present earlier. Luminal invasion of the IVC by a retroperitoneal leiomyosarcoma can produce signs and symptoms relative to distal venous congestion. Suprahepatic invasion may lead to signs and symptoms of Budd-Chiari syndrome or right upper quadrant tenderness, while infrahepatic but suprarenal invasion may present with renal dysfunction; a patient with infrarenal invasion may demonstrate bilateral leg edema.2 Finally, leiomyosarcoma with IVC involvement, regardless of location, has the potential for pulmonary embolism. Contributing to this increased risk is the hypercoagulable state seen in malignancy as well as the propensity for tumors with vascular involvement to form thrombus with subsequent embolization.
Retroperitoneal leiomyosarcomas are most commonly diagnosed using CT or MRI.4 Extravascular growth pattern is the most common, accounting for more than 60% of cases, while extravascular tumor growth with intravascular involvement occurs in approximately 30% of cases.4 Vascular involvement increases the chances of metastasis during diagnosis, with metastases seen in 9% and 23% of extravascular tumors vs tumors with intravascular involvement, respectively.5 Common sites of metastasis include the lung, liver, and peritoneum.
Retroperitoneal leiomyosarcomas generally demonstrate a heterogeneous pattern on CT. In tumors with vascular involvement, a relatively smaller extravascular component may suggest that the tumor originates from the IVC smooth muscle itself, whereas a large extravascular component suggests an extraluminal etiology with secondary IVC involvement. Definitive histological diagnosis is required for planning of neoadjuvant therapy.6 Image-guided biopsy enables the correct diagnosis, including establishing the tumor subtype.7
Surgical resection offers the best opportunity for survival, demonstrating a 5-year disease-free survival of 20-56%.3 In comparison, patients who do not undergo resection typically survive less than 1 year. Resectability in patients with vascular involvement depends on the tumor location and structures involved; IVC reconstruction may be an option.8 In patients demonstrating signs and symptoms from IVC occlusion, including downstream organ dysfunction, bridging stent placement may benefit the patient. The aim of stent placement is to improve quality of life and optimize overall preoperative patient status via restoration of vessel patency.9 Literature on stenting in malignant IVC syndrome is scarce, but data are promising, with reported success rates of 78-100%.10 Stent placement has risks including stent migration and vessel perforation; therefore, this primary palliative management option should be reserved for patients experiencing severe symptoms and decreasing quality of life or who could otherwise become surgical candidates with improvement of status. In patients who are not candidates for surgical resection, treatment options include chemotherapy and radiation therapy.
Conclusion
Retroperitoneal leiomyosarcoma is a rare soft-tissue malignancy with an often delayed presentation. Imaging aids in determining the diagnosis; however, biopsy is typically required. Patients with vascular involvement generally present earlier owing to signs and symptoms; however, these patients are also less likely to be surgical candidates. Bridging stent placement for symptomatic improvement has shown promise in these patients.
References
Citation
IM S, NW R, G V, J E. Retroperitoneal Leiomyosarcoma Invading the Inferior Vena Cava. Appl Radiol. 2024; (4):45 - 47.
doi:10.37549/AR-D-24-0011
August 1, 2024