Renal Angiomyolipoma Secondary to Tuberous Sclerosis
Images



CASE SUMMARY
An 11-year-old with a known history of tuberous sclerosis complex (TSC) and bilateral renal angiomyolypoma (AML), with the dominant lesion on the upper pole of the right kidney, presented for nephrology evaluation.
IMAGING FINDINGS
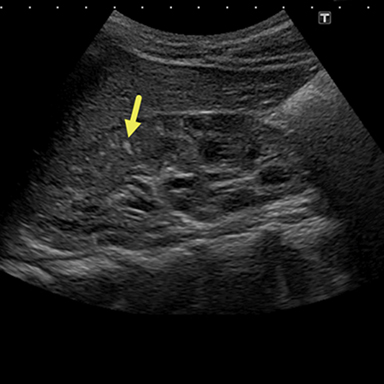
An ultrasound (Figure 1) performed one year prior to presentation demonstrated a heterogenous appearance of both kidneys with multiple small and echogenic lesions scattered throughout each. A 2.7 2.7 2.7 cm mass was present in the right upper renal pole. This mass, slightly echogenic compared to the renal cortex, was determined to be an AML, given the patient’s history of tuberous sclerosis.
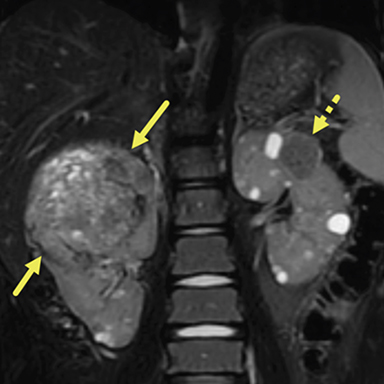
An abdominal MRI (Figure 2) obtained 16 months following the ultrasound demonstrated numerous, bilateral, well-circumscribed fluid signal lesions within the renal parenchyma compatible with cysts. Multiple heterogeneous, fat-containing lesions were also present bilaterally, compatible with AMLs. The large lesion in the right kidney now measured 8.3 5.6 7.3 cm. Gradient echo imaging of this lesion showed areas of susceptibility effect, compatible with hemosiderin deposition from prior hemorrhage. The vasculature medial to the superior aspect of the right kidney was abnormally tortuous and dilated; this was thought to be related to draining veins. All other lesions in both kidneys measured under 3 cm. Follow up MRI performed one year later demonstrated that the AML in the right kidney had grown to 9.6 8.7 6.6 cm. All other AMLs and cystic lesions remained relatively unchanged.
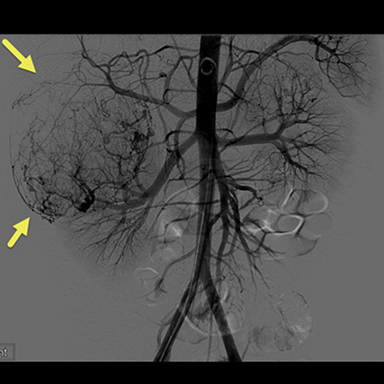
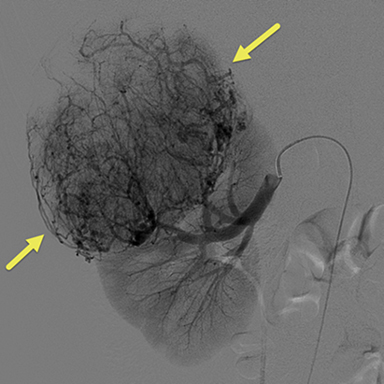
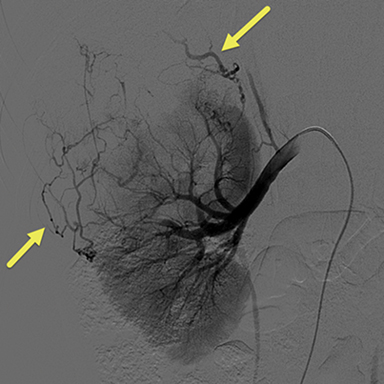
Due to the increasing size of the lesion on the right kidney and the prior hemorrhage, the patient underwent transarterial embolization. Digital subtraction (catheter) arteriography (DSA) (Fig 3) showed extensive tumor vascularity and tumor blushing in the upper pole of the right kidney. The most distal feeding artery branch off the renal artery was subselected coaxially with a micro-cathter and embolization was performed using 150-250 micrometer Contour PVA particles. The microcatheter was withdrawn more proximally into the right renal artery and repeat DSA demonstrated another feeding artery supplying the large upper pole AML. This vessel was also subselected and embolization was performed in the same fashion. After multiple rounds of embolization, DSA revealed sluggish flow in the feeding artery with decreased enhancement in the distribution of the embolized artery. To avoid postembolization syndrome, methylprednisolone and antibiotics were administered immediately before obstruction of flow with particles. The patient was also put on the mTORC1 inhibitor, Everolimus, to help decrease the growth and risk of bleeding of the remaining AMLs.
MRIs were obtained 1 year and 4 years post embolization, and both showing that the right upper pole AML had substantially decreased in size, now measuring approximately 2 3.4 5.4 cm. This lesion remained stable between the two post embolization MRIs. There was heterogeneously hypointense central T1 and T2 signal, reflective of hemorrhagic products. The smaller bilateral AML and cystic lesions remained stable.
DIAGNOSIS
Renal angiomylipoma secondary to tuberous sclerosis
DISCUSSION
TSC is a phenotypically varied, autosomal dominant disease. Multiple organ systems can be involved in the disease process including the following in descending order of morbidity and mortality: brain, kidneys, lungs, heart, liver, adrenal glands, pancreas and spleen.1 TSC has a population prevalence of 5-12 per 100,000 people and an incidence of 1 in 6,000-10,000 live births.1,2 It is caused by a mutation in either TSC1 gene or TSC2 gene, or both, which encode for hamartin and tuberin proteins respectively, allowing for the formation of the TSC1-TSC2 complex. The TSC1-TSC2 complex is an inhibitor of the rapamycin (mTOR) pathway. This pathway plays a role in cellular proliferation, protein synthesis, and metabolism, thus the TSC1-TSC2 complex inhibits tumor growth and proliferation.2,3 Mutations in TSC1 and/or TSC2 lead to a unregulated activation of the mTOR pathway and tumor formation.1
The second International TSC Consensus Conference in 2012 revised criteria used to establish a diagnosis of TSC. The new criteria added the potential for diagnosis based purely on genetic findings.3 While a genetic diagnosis of TSC is now possible, due to gentic variation, many patients are still diagnosed based on clinical findings. These criteria state that for a definitive diagnosis to be made, a patient must have 2 major criteria or 1 major and 2 minor criteria. Major criteria include the most common clinical findings such as subependymal nodules, subependymal giant cell astrocytomas (SEGA), renal AMLs, cardiac rhabdomyomas, lymphangioleiomyomatosis, hypomelanotic macules, angiofibromas, and shagreen patches. The minor criteria include some of the less common clinical findings such as confetti skin lesions, dental enamel pits, and multiple renal cysts.3
Renal AMLs are the most common abdominal manifestation of TSC, present in up to 80% of patients.2,3 AMLs associated with TSC affect both genders equally, are frequently, bilateral, numerous, large, and present early in life.6 There are two major histological types of AMLs associated with TSC: Most common is the classic, which is abundant in fat; less common is the fat-poor or lipid-poor. This subtype is present in over 33% of TSC patients. Compared to patients with sporadic AML, the AMLs in TSC patients have a higher risk of containing an epithelial component.6 mTOR have been shown to decrease the size of brain lesions (tubers, subependymal nodules, and SEGAs), renal AMLs, and renal cystic lesions as well as decrease the frequency of seizures.1,2,4 The benefits of mTor inhibition only last as long as the therapy is continued. Discontinuing mTOR inhibitor therapy leads to rebound growth of these TSC-associated tumors.
On MR imaging, AMLs have a varied appearance based on their lipid content. Classic AMLs appear as a heterogeneous lesion mostly following fat signal. There is near-diffuse lesional saturation when fat saturation is applied. In- and opposed-phase imaging is particularly useful in diagnosing AMLs, with characteristic signal drop-out at the lipid/soft tissue borders. Within small lesions, there is complete loss of signal. In larger lesions, it is typically the soft tissue component that shows signal drop out. Contrast enhancement is not required in patients with known TSC. While AMLs are typically discrete, they can diffusely replace the kidney. Fat-poor lesions are generally hypointense on both T1- and T2-weighted imaging without signal drop out on fat-saturated or opposed-phase images. It can be difficult to identify aneurysms on MRI.
On CT, the appearance of an AML also depends on the fat content. Fat-containing lesions will have areas of low attenuation while fat-poor lesions will have soft tissue attenuation and are difficult to distinguish form the underlying kidney.
As AMLs grow, renal function may begin to decline. Renal failure is a common cause of mortality for adults with TSC. In addition to decreasing renal function, the abnormal vasculature of AMLs may develop macro or microaneurysms, which can hemorrhage, a complication known as Wunderlich syndrome.5,7 Transcatheter embolization is the primary treatment for AMLs in TSC that have either bled or are at high risk of bleeding.
EMBOLIZATION
AMLs require intervention if bleeding occurs. AMLs that are larger than 4 cm are reported to be more likely to spontaneously hemorrhage and may be .5,6 However, in our experience, we have found that large, asymptomatic lesions can be managed conservatively. This is especially true in the era of mTor inhibitors, which help to decrease lesionsal size, inhibit lesional growth, and may decrease the risk of hemorrhage in TSC-related AMLs.
Transarterial embolization is the treatment of choice for AMLs as it allows for more renal sparing than total or partial nephrectomy. The goal of embolization is to occlude the abnormal vasculature and decrease tumor bulk. Transarterial embolization has a 10% complication risk, including accidental embolization of normal tissue, renal infarction, renal failure, and vascular injury.6,7 Post-embolization syndrome is experienced by up to 30% of patients and manifests as leukocytosis, pain, nausea, and fever.6,8 It is theorized to reflect an an inflammatory response to the necrotic tissue after embolization. Steroids are used to help decrease the post-embolization inflammation and thus the risk of this reaction.5
A variety of embolic agents, such as coils, particles, glue, or ethanol, can be used. While none have been shown to be superior, particulate agents are the most commonly used for AML embolization. The popularity of particulate agents may be attributed to their effective occlusion of distal flow and decreased risk of non-target embolization secondary to reflux. This is opposed to coils, which provide only proximal embolization and increase risk of distal collateral formation, and glue or ethanol, which have a higher risk of reflux.8 The success rate of AML embolization is variable due to the heterogenous nature of the tumors. Adipose tissue is more resistant to embolization due to its relative hypovascularity.
CONCLUSION
AMLs are a common manifestation of TSC. Particulate embolization was performed due to the lesion’s large size, evidence of prior hemorrhage, and abnormal, dilated vasculature. mTor inhibitor therapy has helped to revolutionize the treatment of patients with TSC by decreasing the neurologic findings, and the size and number of AMLs. Due to this, embolization of renal AMLs in the setting of TSC has become less common. Our patient has been successfully managed after embolization of his AML. Long-term follow-up imaging has shown a considerable decrease in the size of the lesion. mTor therapy has helped to prevent other lesions from progressing.
REFERENCES
- Morin C, Morin N, Franz D, Krueger D, Trout A, Towbin A. Thoracoabdominal imaging of tuberous sclerosis. Manuscript.
- Curatolo P, Moavero R, de Vries P. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurology. 2015;14: 733-745.
- Portocarrero L, Quental K, Samorano L, Prado de Oliveira Z, and Rivitti-Machado M. Tuberous sclerosis complex: review based on new diagnostic criteria. Anais Brasileriros de Dermatologia. 2018;93(3): 323-331.
- Siroky BJ, Towbin AJ, Trout AT, Schäfer H, Thamann AR, Agricola KD, Tudor C, Capal J, Dixon BP, Krueger DA, Franz DN. Improvement in Renal Cystic Disease of Tuberous Sclerosis Complex After Treatment with Mammalian Target of Rapamycin Inhibitor. J Pediatr. 2017 Aug;187:318-322.
- Bissler J, Racadio J, Donnelley L, Johnson N. Reduction of postembolization syndrome after ablation of renal angiomyolipoma. AJKD. 2002;39(7):966-971.
- Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39(3):588-604.
- Williams J, Racadio J, Johnson N, Donnelley LF, Bissler J. Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. AJKD. 2006;47(1):95-102.
- Kocakgol DO, Cayli E, Oguz S, Dinc H. Selective Arterial Embolization of Giant Renal Angiomyolipoma Associated with Tuberous Sclerosis Complex Using Particular and Liquid Embolic Agents. Eurasian J Med. 2018;50(2):130-133.
Citation
III FD, RL F, RacadioJM, RB T, AJ T.Renal Angiomyolipoma Secondary to Tuberous Sclerosis . Appl Radiol. 2021; (1):44-47.
January 19, 2021